Abstract
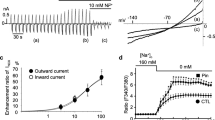
AICAR (5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide) is an adenosine analog which improves the recovery of the heart after ischemia. In some tissues AICAR enters cells and stimulates AMP-activated protein kinase (AMPK). We explored the mechanism of cardioprotection in isolated rat hearts. We confirmed that AICAR (0.5 mM) applied 10 min prior to a 30-min period of ischemia and present throughout ischemia and reperfusion caused a substantial improvement in the recovery of developed pressure on reperfusion. However, adenosine (100 μM) produced no improvement, suggesting that the mechanism of action of AICAR was not increased endogenous adenosine production. Measurements of intracellular sodium concentration ([Na+]i) showed that AICAR prevented the rapid rise of [Na+]i, which normally occurs on reperfusion. Inhibitors of the cardiac sodium–hydrogen exchanger (NHE1) also protect the heart from ischemic damage and also prevent the rapid rise of [Na+]i on reperfusion, suggesting that AICAR might cause the inhibition of NHE1. We tested this possibility on isolated rat ventricular myocytes in which the recovery of pHi after NH4Cl exposure provides a measure of NHE1 activity. AICAR (0.5 μmM) inhibited NHE1 activity in response to an acid load by about 80%. To test whether the AICAR-induced inhibition of NHE1 arose through adenosine, we used the adenosine receptor blocker 8-sulfophenyltheophylline (8-SPT) and found that it had no measureable effect. To test whether the AICAR-induced inhibition of NHE1 might occur through the activation of AMPK, we measured the activity of two isoforms of AMPK. Surprisingly, activity was reduced, whereas in many other tissues AICAR increases AMPK activity. Furthermore, this effect of AMPK was blocked by 8-SPT, suggesting that the inhibition of AMPK arose through an adenosine-receptor-related pathway. We conclude that AICAR inhibits NHE1 through an unidentified pathway. This inhibition may make a contribution to the cardioprotective effects of AICAR.





Similar content being viewed by others
References
Allen DG, Xiao XH (2001) Letter to the editor; Na+ entry during ischemia, reperfusion and preconditioning. Cardiovasc Res 50:164–166
An J, Varadarajan SG, Camara A, Chen Q, Novalija E, Gross GJ et al (2001) Blocking Na(+)/H(+) exchange reduces [Na(+)](i) and [Ca(2+)](i) load after ischemia and improves function in intact hearts. Am J Physiol Heart Circ Physiol 281:H2398–H2409
Avkiran M, Yokoyama H (2000) Adenosine A(1) receptor stimulation inhibits alpha(1)-adrenergic activation of the cardiac sarcolemmal Na(+)/H(+) exchanger. Br J Pharmacol 131:659–662
Bolli R, Marban E (1999) Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79:609–634
Cairns SP, Westerblad H, Allen DG (1993) Changes in myoplasmic pH and calcium concentration during exposure to lactate in isolated rat ventricular myocytes. J Physiol 464:561–574
Cave AC, Collis CS, Downey JM, Hearse DJ (1993) Improved functional recovery by ischaemic preconditioning is not mediated by adenosine in the globally ischaemic isolated rat heart. Cardiovasc Res 27:663–668
Chen Z, Heierhorst J, Mann RJ, Mitchelhill KI, Michell BJ, Witters LA et al (1999) Expression of the AMP-activated protein kinase beta1 and beta2 subunits in skeletal muscle. FEBS Lett 460:343–348
Cook MA, Karmazyn M (1996) Cardioprotective actions of adenosine and adenosine analogs. In: Karmazyn M (ed) Myocardial ischemia: mechanisms, reperfusion, protection. Birkäuser, Basel, pp 325–344
Corton JM, Gillespie JG, Hawley SA, Hardie DG (1995) 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229:558–565
da Silva CG, Jarzyna R, Specht A, Kaczmarek E (2006) Extracellular nucleotides and adenosine independently activate AMP-activated protein kinase in endothelial cells: involvement of P2 receptors and adenosine transporters. Circ Res 98:e39–e47
Elliott AC, Smith GL, Eisner DA, Allen DG (1992) Metabolic changes during ischaemia and their role in contractile failure in isolated ferret hearts. J Physiol 454:467–490
Fralix TA, Murphy E, London RE, Steenbergen C (1993) Protective effects of adenosine in the perfused rat heart: changes in metabolism and intracellular ion homeostasis. Am J Physiol 264:C986–C994
Galinanes M, Bullough D, Mullane KM, Hearse DJ (1992) Sustained protection by acadesine against ischemia- and reperfusion-induced injury. Studies in the transplanted rat heart. Circulation 86:589–597
Galinanes M, Mullane KM, Bullough D, Hearse DJ (1992) Acadesine and myocardial protection. Studies of time of administration and dose-response relations in the rat. Circulation 86:598–608
Ganote CE, Armstrong SC (2000) Adenosine and preconditioning in the rat heart. Cardiovasc Res 45:134–140
Gruber HE, Hoffer ME, McAllister DR, Laikind PK, Lane TA, Schmid-Schoenbein GW et al (1989) Increased adenosine concentration in blood from ischemic myocardium by AICA riboside. Effects on flow, granulocytes, and injury. Circulation 80:1400–1411
Henin N, Vincent MF, Van den Berghe G (1996) Stimulation of rat liver AMP-activated protein kinase by AMP analogues. Biochim Biophys Acta 1290:197–203
Javaux F, Vincent MF, Wagner DR, Van den Berghe G (1995) Cell-type specificity of inhibition of glycolysis by 5-amino-4-imidazolecarboxamide riboside. Lack of effect in rabbit cardiomyocytes and human erythrocytes, and inhibition in FTO-2B rat hepatoma cells. Biochem J 305(Pt 3):913–919
Karmazyn M (1988) Amiloride enhances postischemic ventricular recovery: possible role of Na+–H+ exchange. Am J Physiol 255:H608–H615
Karmazyn M, Gan XT, Humphreys RA, Yoshida H, Kusumoto K (1999) The myocardial Na+–H+ exchange: structure, regulation, and its role in heart disease. Circ Res 85:777–786
Kitakaze M, Takashima S, Minamino T, Node K, Shinozaki Y, Mori H et al (1999) Improvement by 5-amino-4-imidazole carboxamide riboside of the contractile dysfunction that follows brief periods of ischemia through increases in ecto-5-nucleotidase activity and adenosine release in canine hearts. Jpn Circ J 63:542–553
Kleyman TR, Cragoe EJ (1988) Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105:1–21
Lasley RD, Hegge JO, Noble MA, Mentzer RM Jr (1998) Comparison of interstitial fluid and coronary venous adenosine levels in in vivo porcine myocardium. J Mol Cell Cardiol 30:1137–1147
Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD (1999) Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol 517:159–180
Li Y, Kloner RA (1993) The cardioprotective effects of ischemic ‘preconditioning’ are not mediated by adenosine receptors in rat hearts. Circulation 87:1642–1648
Liem DA, te Lintel HM, Manintveld OC, Boomsma F, Verdouw PD, Duncker DJ (2005) Myocardium tolerant to an adenosine-dependent ischemic preconditioning stimulus can still be protected by stimuli that employ alternative signaling pathways. Am J Physiol Heart Circ Physiol 288:H1165–H1172
Longnus SL, Wambolt RB, Parsons HL, Brownsey RW, Allard MF (2003) 5-Aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside (AICAR) stimulates myocardial glycogenolysis by allosteric mechanisms. Am J Physiol Regul Integr Comp Physiol 284:R936–R944
Mangano DT (1997) Effects of acadesine on myocardial infarction, stroke, and death following surgery. A meta-analysis of the 5 international randomized trials. The Multicenter Study of Perioperative Ischemia (McSPI) Research Group. JAMA 277:325–332
Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF et al (2000) Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 10:1247–1255
Mauser M, Hoffmeister HM, Nienaber C, Schaper W (1985) Influence of ribose, adenosine, and “AICAR” on the rate of myocardial adenosine triphosphate synthesis during reperfusion after coronary artery occlusion in the dog. Circ Res 56:220–230
Menasche P, Jamieson WR, Flameng W, Davies MK (1995) Acadesine: a new drug that may improve myocardial protection in coronary artery bypass grafting. Results of the first international multicenter study. Multinational Acadesine Study Group. J Thorac Cardiovasc Surg 110:1096–1106
Meng HP, Lonsberry BB, Pierce GN (1991) Influence of perfusate pH on the postischemic recovery of cardiac contractile function: involvement of sodium-hydrogen exchange. J Pharmacol Exp Ther 258:772–777
Mullane K (1993) Acadesine: the prototype adenosine regulating agent for reducing myocardial ischaemic injury. Cardiovasc Res 27:43–47
Nakai T, Kano S, Satoh K, Hoshi K, Ichihara K (1996) Effects of adenine nucleotide analogues on myocardial dysfunction during reperfusion after ischemia in dogs. J Cardiovasc Pharmacol 28:264–270
Park CO, Xiao XH, Allen DG (1999) Changes in intracellular sodium and pH in the rat heart during ischaemia: role of the Na+/H+ exchanger. Am J Physiol 276:H1581–H1590
Peart J, Matherne GP, Cerniway RJ, Headrick JP (2001) Cardioprotection with adenosine metabolism inhibitors in ischemic-reperfused mouse heart. Cardiovasc Res 52:120–129
Russell RR III, Bergeron R, Shulman GI, Young LH (1999) Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol 277:H643–H649
Scholz W, Albus U, Counillon L, Gogelein H, Lang HJ, Linz W et al (1995) Protective effects of HOE642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc Res 29:260–268
Slepkov E, Fliegel L (2002) Structure and function of the NHE1 isoform of the Na+/H+ exchanger. Biochem Cell Biol 80:499–508
Swain JL, Hines JJ, Sabina RL, Holmes EW (1982) Accelerated repletion of ATP and GTP pools in postischemic canine myocardium using a precursor of purine de novo synthesis. Circ Res 51:102–105
Tani M, Neely JR (1989) Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+–Na+ and Na+–Ca2+ exchange. Circ Res 65:1045–1056
Xiao XH, Allen DG (1999) Role of the Na+/H+ exchanger during ischemia and preconditioning in the isolated rat heart. Circ Res 85:723–730
Xiao X, Allen DG (2000) Activity of the Na+/H+ exchanger is critical to reperfusion damage and preconditioning in the isolated rat heart. Cardiovasc Res 48:244–253
Xiao XH, Allen DG (2002) The role of endogenous angiotensin II in ischemia, reperfusion and preconditioning of the isolated rat heart. Pflügers Arch 445:643–650
Xiao XH, Allen DG (2003) The cardioprotective effects of Na+/H+ exchange inhibition and mitochondrial KATP channel activation are additive in the isolated rat heart. Pflugers Arch 447:272–279
Zhao ZQ, Williams MW, Sato H, Hudspeth DA, McGee DS, Vinten-Johansen J et al (1995) Acadesine reduces myocardial infarct size by an adenosine mediated mechanism. Cardiovasc Res 29:495–505
Acknowledgement
This study was supported by the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moopanar, T.R., Xiao, XH., Jiang, L. et al. AICAR inhibits the Na+/H+ exchanger in rat hearts—possible contribution to cardioprotection. Pflugers Arch - Eur J Physiol 453, 147–156 (2006). https://doi.org/10.1007/s00424-006-0124-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0124-z