Abstract
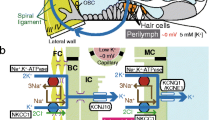
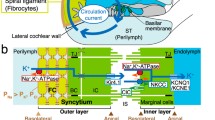
Cochlear endolymph, an extracellular solution containing 150 mM K+, exhibits a positive potential of +80 mV. This is called the endocochlear potential (EP) and is essential for audition. The mechanism responsible for formation of the EP has been an enigma for the half century since its first measurement. A key element is the stria vascularis, which displays a characteristic tissue structure and expresses multiple ion-transport apparatus. The stria comprises two epithelial layers: a layer of marginal cells and one composed of intermediate and basal cells. Between the two layers lies an extracellular space termed the intrastrial space (IS), which is thus surrounded by the apical membranes of intermediate cells and the basolateral membranes of marginal cells. The fluid in the IS exhibits a low concentration of K+ and a positive potential similar to the EP. We have demonstrated that the IS is electrically isolated from the neighboring extracellular fluids, perilymph, and endolymph, which allows the IS to sustain its positive potential. This IS potential is generated by K+ diffusion across the apical membranes of intermediate cells, where inwardly rectifying Kir4.1 channels are localized. The low K+ concentration in the IS, which is mandatory for the large K+-diffusion potential, is maintained by Na+,K+-ATPases and Na+,K+,2Cl−-cotransporters expressed at the basolateral membranes of marginal cells. An additional K+-diffusion potential formed by KCNQ1/KCNE1-K+ channels at the apical membranes of marginal cells also contributes to the EP. Therefore, the EP depends on an electrically isolated space and two K+-diffusion potentials in the stria vascularis.






Similar content being viewed by others
References
Ando M, Takeuchi S (1999) Immunological identification of an inward rectifier K+ channel (Kir4.1) in the intermediate cell (melanocyte) of the cochlear stria vascularis of gerbils and rats. Cell Tissue Res 298:179–183
Ando M, Takeuchi S (2000) mRNA encoding 'ClC-K1, a kidney Cl− channel' is expressed in marginal cells of the stria vascularis of rat cochlea: its possible contribution to Cl− currents. Neurosci Lett 284:171–174
Beisel KW, Rocha-Sanchez SM, Morris KA, Nie L, Feng F, Kachar B, Yamoah EN, Fritzsch B (2005) Differential expression of KCNQ4 in inner hair cells and sensory neurons is the basis of progressive high-frequency hearing loss. J Neurosci 25:9285–9293
Birkenhager R, Otto E, Schurmann MJ, Vollmer M, Ruf EM, Maier-Lutz I, Beekmann F, Fekete A, Omran H, Feldmann D, Milford DV, Jeck N, Konrad M, Landau D, Knoers NV, Antignac C, Sudbrak R, Kispert A, Hildebrandt F (2001) Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet 29:310–314
Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van't Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R (2009) Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360:1960–1970
Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, Jentsch TJ (2002) Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature 416:874–878
Boettger T, Rust MB, Maier H, Seidenbecher T, Schweizer M, Keating DJ, Faulhaber J, Ehmke H, Pfeffer C, Scheel O, Lemcke B, Horst J, Leuwer R, Pape HC, Volkl H, Hubner CA, Jentsch TJ (2003) Loss of K-Cl co-transporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. Embo J 22:5422–5434
Cable J, Steel KP (1998) Combined cochleo-saccular and neuroepithelial abnormalities in the Varitint-waddler-J (VaJ) mouse. Hear Res 123:125–136
Casimiro MC, Knollmann BC, Ebert SN, Vary JC Jr, Greene AE, Franz MR, Grinberg A, Huang SP, Pfeifer K (2001) Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen Syndrome. Proc Natl Acad Sci U S A 98:2526–2531
Chan DK, Hudspeth AJ (2005) Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro. Nat Neurosci 8:149–155
Cohen-Salmon M, Regnault B, Cayet N, Caille D, Demuth K, Hardelin JP, Janel N, Meda P, Petit C (2007) Connexin30 deficiency causes instrastrial fluid–blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci U S A 104:6229–6234
Crouch JJ, Sakaguchi N, Lytle C, Schulte BA (1997) Immunohistochemical localization of the Na-K-Cl co-transporter (NKCC1) in the gerbil inner ear. J Histochem Cytochem 45:773–778
Cuajungco MP, Grimm C, Heller S (2007) TRP channels as candidates for hearing and balance abnormalities in vertebrates. Biochim Biophys Acta 1772:1022–1027
Dallos P, Fakler B (2002) Prestin, a new type of motor protein. Nat Rev Mol Cell Biol 3:104–111
Delpire E, Lu J, England R, Dull C, Thorne T (1999) Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet 22:192–195
Di Palma F, Belyantseva IA, Kim HJ, Vogt TF, Kachar B, Noben-Trauth K (2002) Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc Natl Acad Sci U S A 99:14994–14999
Dixon MJ, Gazzard J, Chaudhry SS, Sampson N, Schulte BA, Steel KP (1999) Mutation of the Na-K-Cl co-transporter gene Slc12a2 results in deafness in mice. Hum Mol Genet 8:1579–1584
Dulon D, Sugasawa M, Blanchet C, Erostegui C (1995) Direct measurements of Ca2+-activated K+ currents in inner hair cells of the guinea-pig cochlea using photolabile Ca2+ chelators. Pflugers Arch 430:365–373
Estevez R, Boettger T, Stein V, Birkenhager R, Otto E, Hildebrandt F, Jentsch TJ (2001) Barttin is a Cl− channel beta-subunit crucial for renal Cl− reabsorption and inner ear K+ secretion. Nature 414:558–561
Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE (1999) Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem 274:26946–26955
Geleoc GS, Lennan GW, Richardson GP, Kros CJ (1997) A quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc Biol Sci 264:611–621
Gow A, Davies C, Southwood CM, Frolenkov G, Chrustowski M, Ng L, Yamauchi D, Marcus DC, Kachar B (2004) Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. J Neurosci 24:7051–7062
Grimm C, Cuajungco MP, van Aken AF, Schnee M, Jors S, Kros CJ, Ricci AJ, Heller S (2007) A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc Natl Acad Sci U S A 104:19583–19588
Hama K, Saito K (1977) Gap junctions between the supporting cells in some acoustico-vestibular receptors. J Neurocytol 6:1–12
Hibino H, Higashi-Shingai K, Fujita A, Iwai K, Ishii M, Kurachi Y (2004) Expression of an inwardly rectifying K+ channel, Kir5.1, in specific types of fibrocytes in the cochlear lateral wall suggests its functional importance in the establishment of endocochlear potential. Eur J Neurosci 19:76–84
Hibino H, Horio Y, Inanobe A, Doi K, Ito M, Yamada M, Gotow T, Uchiyama Y, Kawamura M, Kubo T, Kurachi Y (1997) An ATP-dependent inwardly rectifying potassium channel, KAB-2 (Kir4.1), in cochlear stria vascularis of inner ear: its specific subcellular localization and correlation with the formation of endocochlear potential. J Neurosci 17:4711–4721
Hibino H, Kurachi Y (2006) Molecular and physiological bases of the K+ circulation in the mammalian inner ear. Physiology (Bethesda) 21:336–345
Hinojosa R, Rodriguez-Echandia EL (1966) The fine structure of the stria vascularis of the cat inner ear. Am J Anat 118:631–663
Hudspeth AJ (1989) How the ear's works work. Nature 341:397–404
Hudspeth AJ (1997) How hearing happens. Neuron 19:947–950
Hudspeth AJ (2008) Making an effort to listen: mechanical amplification in the ear. Neuron 59:530–545
Hudspeth AJ, Corey DP (1977) Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A 74:2407–2411
Ikeda K, Morizono T (1989) Electrochemical profiles for monovalent ions in the stria vascularis: cellular model of ion transport mechanisms. Hear Res 39:279–286
Iwano T, Yamamoto A, Omori K, Akayama M, Kumazawa T, Tashiro Y (1989) Quantitative immunocytochemical localization of Na+, K+-ATPase alpha-subunit in the lateral wall of rat cochlear duct. J Histochem Cytochem 37:353–363
Jahnke K (1975) The fine structure of freeze-fractured intercellular junctions in the guinea pig inner ear. Acta Otolaryngol Suppl 336:1–40
Keating MT, Sanguinetti MC (2001) Molecular and cellular mechanisms of cardiac arrhythmias. Cell 104:569–580
Kennedy HJ, Evans MG, Crawford AC, Fettiplace R (2003) Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci 6:832–836
Kerr TP, Ross MD, Ernst SA (1982) Cellular localization of Na+, K+-ATPase in the mammalian cochlear duct: significance for cochlear fluid balance. Am J Otolaryngol 3:332–338
Kharkovets T, Dedek K, Maier H, Schweizer M, Khimich D, Nouvian R, Vardanyan V, Leuwer R, Moser T, Jentsch TJ (2006) Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. Embo J 25:642–652
Kikuchi T, Adams JC, Miyabe Y, So E, Kobayashi T (2000) Potassium ion recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med Electron Microsc 33:51–56
Kikuchi T, Kimura RS, Paul DL, Adams JC (1995) Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol (Berl) 191:101–118
Kitajiri S, Miyamoto T, Mineharu A, Sonoda N, Furuse K, Hata M, Sasaki H, Mori Y, Kubota T, Ito J, Furuse M, Tsukita S (2004) Compartmentalization established by claudin-11-based tight junctions in stria vascularis is required for hearing through generation of endocochlear potential. J Cell Sci 117:5087–5096
Kitajiri SI, Furuse M, Morita K, Saishin-Kiuchi Y, Kido H, Ito J, Tsukita S (2004) Expression patterns of claudins, tight junction adhesion molecules, in the inner ear. Hear Res 187:25–34
Konishi T, Butler RA, Fernandez C (1961) Effect of anoxia on cochlear potentials. J Acoust Soc Amer 33:349–390
Konishi T, Hamrick PE, Walsh PJ (1978) Ion transport in guinea pig cochlea. I. Potassium and sodium transport. Acta Otolaryngol 86:22–34
Konishi T, Mendelsohn M (1970) Effect of ouabain on cochlear potentials and endolymph composition in guinea pigs. Acta Otolaryngol 69:192–199
Kros CJ, Ruppersberg JP, Rusch A (1998) Expression of a potassium current in inner hair cells during development of hearing in mice. Nature 394:281–284
Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch TJ (1999) KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 96:437–446
Kuijpers W, Bonting SL (1970) The cochlear potentials. I. The effect of ouabain on the cochlear potentials of the guinea pig. Pflugers Arch 320:348–358
Kuijpers W, Bonting SL (1970) The cochlear potentials. II. The nature of the cochlear endolymphatic resting potential. Pflugers Arch 320:359–372
Kusakari J, Ise I, Comegys TH, Thalmann I, Thalmann R (1978) Effect of ethacrynic acid, furosemide, and ouabain upon the endolymphatic potential and upon high energy phosphates of the stria vascularis. Laryngoscope 88:12–37
Langer P, Grunder S, Rusch A (2003) Expression of Ca2+-activated BK channel mRNA and its splice variants in the rat cochlea. J Comp Neurol 455:198–209
Lautermann J, Frank HG, Jahnke K, Traub O, Winterhager E (1999) Developmental expression patterns of connexin26 and -30 in the rat cochlea. Dev Genet 25:306–311
Lautermann J, ten Cate WJ, Altenhoff P, Grummer R, Traub O, Frank H, Jahnke K, Winterhager E (1998) Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res 294:415–420
Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103:525–535
Mammano F, Bortolozzi M, Ortolano S, Anselmi F (2007) Ca2+ signaling in the inner ear. Physiology (Bethesda) 22:131–144
Marcus DC (1984) Characterization of potassium permeability of cochlear duct by perilymphatic perfusion of barium. Am J Physiol 247:C240–C246
Marcus DC, Liu J, Wangemann P (1994) Transepithelial voltage and resistance of vestibular dark cell epithelium from the gerbil ampulla. Hear Res 73:101–108
Marcus DC, Marcus NY, Thalmann R (1981) Changes in cation contents of stria vascularis with ouabain and potassium-free perfusion. Hear Res 4:149–160
Marcus DC, Rokugo M, Thalmann R (1985) Effects of barium and ion substitutions in artificial blood on endocochlear potential. Hear Res 17:79–86
Marcus DC, Wu T, Wangemann P, Kofuji P (2002) KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol Cell Physiol 282:C403–C407
Melichar I, Syka J (1977) Time course of anoxia-induced K+ concentration changes in the cochlea measured with K+ specific microelectrodes. Pflugers Arch 372:207–213
Melichar I, Syka J (1987) Electrophysiological measurements of the stria vascularis potentials in vivo. Hear Res 25:35–43
Minowa O, Ikeda K, Sugitani Y, Oshima T, Nakai S, Katori Y, Suzuki M, Furukawa M, Kawase T, Zheng Y, Ogura M, Asada Y, Watanabe K, Yamanaka H, Gotoh S, Nishi-Takeshima M, Sugimoto T, Kikuchi T, Takasaka T, Noda T (1999) Altered cochlear fibrocytes in a mouse model of DFN3 nonsyndromic deafness. Science 285:1408–1411
Mori Y, Watanabe M, Inui T, Nimura Y, Araki M, Miyamoto M, Takenaka H, Kubota T (2009) Ca2+ regulation of endocochlear potential in marginal cells. J Physiol Sci 59:355–365
Nagata K, Zheng L, Madathany T, Castiglioni AJ, Bartles JR, Garcia-Anoveros J (2008) The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proc Natl Acad Sci U S A 105:353–358
Neyroud N, Tesson F, Denjoy I, Leibovici M, Donger C, Barhanin J, Faure S, Gary F, Coumel P, Petit C, Schwartz K, Guicheney P (1997) A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange–Nielsen cardioauditory syndrome. Nat Genet 15:186–189
Nicolas M, Dememes D, Martin A, Kupershmidt S, Barhanin J (2001) KCNQ1/KCNE1 potassium channels in mammalian vestibular dark cells. Hear Res 153:132–145
Nin F, Hibino H, Doi K, Suzuki T, Hisa Y, Kurachi Y (2008) The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc Natl Acad Sci U S A 105:1751–1756
Offner FF, Dallos P, Cheatham MA (1987) Positive endocochlear potential: mechanism of production by marginal cells of stria vascularis. Hear Res 29:117–124
Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B (2000) Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron 26:595–601
Phippard D, Lu L, Lee D, Saunders JC, Crenshaw EB 3rd (1999) Targeted mutagenesis of the POU-domain gene Brn4/Pou3f4 causes developmental defects in the inner ear. J Neurosci 19:5980–5989
Rickheit G, Maier H, Strenzke N, Andreescu CE, De Zeeuw CI, Muenscher A, Zdebik AA, Jentsch TJ (2008) Endocochlear potential depends on Cl− channels: mechanism underlying deafness in Bartter syndrome IV. EMBO J 27:2907–2917
Rivas A, Francis HW (2005) Inner ear abnormalities in a Kcnq1 (Kvlqt1) knockout mouse: a model of Jervell and Lange–Nielsen syndrome. Otol Neurotol 26:415–424
Ruttiger L, Sausbier M, Zimmermann U, Winter H, Braig C, Engel J, Knirsch M, Arntz C, Langer P, Hirt B, Muller M, Kopschall I, Pfister M, Munkner S, Rohbock K, Pfaff I, Rusch A, Ruth P, Knipper M (2004) Deletion of the Ca2+-activated potassium (BK) α-subunit but not the BKβ1-subunit leads to progressive hearing loss. Proc Natl Acad Sci U S A 101:12922–12927
Sage CL, Marcus DC (2001) Immunolocalization of ClC-K chloride channel in strial marginal cells and vestibular dark cells. Hear Res 160:1–9
Sakagami M, Fukazawa K, Matsunaga T, Fujita H, Mori N, Takumi T, Ohkubo H, Nakanishi S (1991) Cellular localization of rat Isk protein in the stria vascularis by immunohistochemical observation. Hear Res 56:168–172
Sakaguchi N, Crouch JJ, Lytle C, Schulte BA (1998) Na-K-Cl cotransporter expression in the developing and senescent gerbil cochlea. Hear Res 118:114–122
Salt AN, Melichar I, Thalmann R (1987) Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope 97:984–991
Schlingmann KP, Konrad M, Jeck N, Waldegger P, Reinalter SC, Holder M, Seyberth HW, Waldegger S (2004) Salt wasting and deafness resulting from mutations in two chloride channels. N Engl J Med 350:1314–1319
Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP (2009) Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A 106:5842–5847
Schulte BA, Adams JC (1989) Distribution of immunoreactive Na+, K+-ATPase in gerbil cochlea. J Histochem Cytochem 37:127–134
Schulte BA, Steel KP (1994) Expression of alpha and beta subunit isoforms of Na, K-ATPase in the mouse inner ear and changes with mutations at the Wv or Sld loci. Hear Res 78:65–76
Schulze-Bahr E, Wang Q, Wedekind H, Haverkamp W, Chen Q, Sun Y, Rubie C, Hordt M, Towbin JA, Borggrefe M, Assmann G, Qu X, Somberg JC, Breithardt G, Oberti C, Funke H (1997) KCNE1 mutations cause Jervell and Lange–Nielsen syndrome. Nat Genet 17:267–268
Sellick PM, Johnstone BM (1972) The electrophysiology of the saccule. Pflugers Arch 336:28–34
Shen Z, Marcus DC (1998) Divalent cations inhibit IsK/KvLQT1 channels in excised membrane patches of strial marginal cells. Hear Res 123:157–167
Skinner LJ, Enee V, Beurg M, Jung HH, Ryan AF, Hafidi A, Aran JM, Dulon D (2003) Contribution of BK Ca2+-activated K+ channels to auditory neurotransmission in the guinea pig cochlea. J Neurophysiol 90:320–332
Spicer SS, Schulte BA (1996) The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hear Res 100:80–100
Spicer SS, Schulte BA (2005) Novel structures in marginal and intermediate cells presumably relate to functions of apical versus basal strial strata. Hear Res 200:87–101
Sterkers O, Saumon G, Tran Ba Huy P, Amiel C (1982) K, Cl, and H2O entry in endolymph, perilymph, and cerebrospinal fluid of the rat. Am J Physiol 243:F173–F180
Sunose H, Ikeda K, Saito Y, Nishiyama A, Takasaka T (1993) Nonselective cation and Cl channels in luminal membrane of the marginal cell. Am J Physiol 265:C72–C78
Sunose H, Ikeda K, Suzuki M, Takasaka T (1994) Voltage-activated K channel in luminal membrane of marginal cells of stria vascularis dissected from guinea pig. Hear Res 80:86–92
Takeuchi S, Ando M (1998) Dye-coupling of melanocytes with endothelial cells and pericytes in the cochlea of gerbils. Cell Tissue Res 293:271–275
Takeuchi S, Ando M (1998) Inwardly rectifying K+ currents in intermediate cells in the cochlea of gerbils: a possible contribution to the endocochlear potential. Neurosci Lett 247:175–178
Takeuchi S, Ando M (1999) Voltage-dependent outward K+ current in intermediate cell of stria vascularis of gerbil cochlea. Am J Physiol 277:C91–C99
Takeuchi S, Ando M, Kakigi A (2000) Mechanism generating endocochlear potential: role played by intermediate cells in stria vascularis. Biophys J 79:2572–2582
Takeuchi S, Ando M, Kozakura K, Saito H, Irimajiri A (1995) Ion channels in basolateral membrane of marginal cells dissociated from gerbil stria vascularis. Hear Res 83:89–100
Takeuchi S, Marcus DC, Wangemann P (1992) Ca2+-activated nonselective cation, maxi K+ and Cl− channels in apical membrane of marginal cells of stria vascularis. Hear Res 61:86–96
Takumida M, Kubo N, Ohtani M, Suzuka Y, Anniko M (2005) Transient receptor potential channels in the inner ear: presence of transient receptor potential channel subfamily 1 and 4 in the guinea pig inner ear. Acta Otolaryngol 125:929–934
Tasaki I, Spyropoulos CS (1959) Stria vascularis as source of endocochlear potential. J Neurophysiol 22:149–155
ten Cate WJ, Curtis LM, Rarey KE (1994) Na, K-ATPase α and β subunit isoform distribution in the rat cochlear and vestibular tissues. Hear Res 75:151–160
Teubner B, Michel V, Pesch J, Lautermann J, Cohen-Salmon M, Sohl G, Jahnke K, Winterhager E, Herberhold C, Hardelin JP, Petit C, Willecke K (2003) Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet 12:13–21
Tranebjaerg L, Bathen J, Tyson J, Bitner-Glindzicz M (1999) Jervell and Lange–Nielsen syndrome: a Norwegian perspective. Am J Med Genet 89:137–146
Vetter DE, Mann JR, Wangemann P, Liu J, McLaughlin KJ, Lesage F, Marcus DC, Lazdunski M, Heinemann SF, Barhanin J (1996) Inner ear defects induced by null mutation of the isk gene. Neuron 17:1251–1264
Vilstrup G (1955) The vitreous body and the endolymph as two related gelatinous substances; comparison of the two substances with reference to their hyaluronic acid and protein contents; preliminary report. Acta Ophthalmol (Copenh) 33:13–15
Vollrath MA, Kwan KY, Corey DP (2007) The micromachinery of mechanotransduction in hair cells. Annu Rev Neurosci 30:339–365
Von Bekesy G (1952) DC resting potentials inside the cochlear partition. J Acoust Soc Amer 24:72–76
Von Bekesy G (1952) Resting potentials inside the cochlear partition of the guinea pig. Nature 169:241–242
Wada J, Kambayashi J, Marcus DC, Thalmann R (1979) Vascular perfusion of the cochlea: effect of potassium-free and rubidium-substituted media. Arch Otorhinolaryngol 225:79–81
Wada J, Paloheimo S, Thalmann I, Bohne BA, Thalmann R (1979) Maintenance of cochlear function with artificial oxygen carriers. Laryngoscope 89:1457–1473
Wang Q, Bowles NE, Towbin JA (1998) The molecular basis of long QT syndrome and prospects for therapy. Mol Med Today 4:382–388
Wangemann P (1995) Comparison of ion transport mechanisms between vestibular dark cells and strial marginal cells. Hear Res 90:149–157
Wangemann P (2002) K+ cycling and the endocochlear potential. Hear Res 165:1–9
Wangemann P (2006) Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol 576:11–21
Wangemann P, Itza EM, Albrecht B, Wu T, Jabba SV, Maganti RJ, Lee JH, Everett LA, Wall SM, Royaux IE, Green ED, Marcus DC (2004) Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med 2:30
Wangemann P, Liu J, Marcus DC (1995) Ion transport mechanisms responsible for K+ secretion and the transepithelial voltage across marginal cells of stria vascularis in vitro. Hear Res 84:19–29
Xu H, Delling M, Li L, Dong X, Clapham DE (2007) Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint-waddler mice. Proc Natl Acad Sci U S A 104:18321–18326
Zidanic M, Brownell WE (1990) Fine structure of the intracochlear potential field. I. The silent current. Biophys J 57:1253–1268
Acknowledgments
We thank Dr. Bernd Nilius (KU Leuven) for providing us with an opportunity to write this review article and Dr. A. J. Hudspeth (The Rockefeller University) for his critical reading of the text. HH and YK are supported by the following research grants and funds: Grant-in-Aid for Scientific Research on Priority Areas 17081012 (to HH), Grant-in-Aid for Young Scientists (B) 19790188 (to HH), the Global COE Program “in silico medicine” at Osaka University (to HH, FN, and YK), and a grant for “Research and Development of Next-Generation Integrated Life Simulation Software” (to YK), from the Ministry of Education, Culture, Sport, Science and Technology of Japan, Senri Life Science Foundation (to HH), Japan Foundation for Applied Enzymology (to HH), and the Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical care (to HH).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hibino, H., Nin, F., Tsuzuki, C. et al. How is the highly positive endocochlear potential formed? The specific architecture of the stria vascularis and the roles of the ion-transport apparatus. Pflugers Arch - Eur J Physiol 459, 521–533 (2010). https://doi.org/10.1007/s00424-009-0754-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-009-0754-z