Abstract
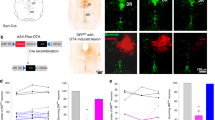
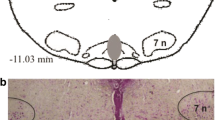
Serotonergic (5-HT) neurons in the nucleus raphe obscurus (ROb) are involved in the respiratory control network. However, it is not known whether ROb 5-HT neurons play a role in the functional interdependence between central and peripheral chemoreceptors. Therefore, we investigated the role of ROb 5-HT neurons in the ventilatory responses to CO2 and their putative involvement in the central–peripheral CO2 chemoreceptor interaction in unanaesthetised rats. We used a chemical lesion specific for 5-HT neurons (anti-SERT-SAP) of the ROb in animals with the carotid body (CB) intact or removed (CBR). Pulmonary ventilation (V E), body temperature and the arterial blood gases were measured before, during and after a hypercapnic challenge (7% CO2). The lesion of ROb 5-HT neurons alone (CB intact) or the lesion of 5-HT neurons of ROb+CBR did not affect baseline V E during normocapnic condition. Killing ROb 5-HT neurons (CB intact) significantly decreased the ventilatory response to hypercapnia (p < 0.05). The reduction in CO2 sensitivity was approximately 15%. When ROb 5-HT neurons lesion was combined with CBR (anti-SERT-SAP+CBR), the V E response to hypercapnia was further decreased (−31.2%) compared to the control group. The attenuation of CO2 sensitivity was approximately 30%, and it was more pronounced than the sum of the individual effects of central (ROb lesion; −12.3%) or peripheral (CBR; −5.5%) treatments. Our data indicate that ROb 5-HT neurons play an important role in the CO2 drive to breathing and may act as an important element in the central–peripheral chemoreception interaction to CO2 responsiveness.





Similar content being viewed by others
References
Barros RC, Bonagamba LG, Okamoto-Canesin R, de Oliveira M, Branco LG, Machado BH (2002) Cardiovascular responses to chemoreflex activation with potassium cyanide or hypoxic hypoxia in awake rats. Auton Neurosci 97:110–115
Biancardi V, Bícego KC, Almeida MC, Gargaglioni LH (2008) Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflugers Arch 455:1119–1128
Blain GM, Smith CA, Henderson KS, Dempsey JA (2009) Contribution of the carotid body chemoreceptors to eupneic ventilation in the intact, unanesthetized dog. J Appl Physiol 106:1564–1573
Blain GM, Smith CA, Henderson KS, Dempsey JA (2010) Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J Physiol 588:2455–2471
Bou-Flores C, Lajard AM, Monteau R, De Maeyer E, Seif I, Lanoir J, Hilaire G (2000) Abnormal phrenic motoneuron activity and morphology in neonatal monoamine oxidase A-deficient transgenic mice: possible role of a serotonin excess. J Neurosci 20:4646–4656
Cao Y, Fujito Y, Matsuyama K, Aoki M (2006) Effects of electrical stimulation of the medullary raphe nuclei on respiratory movement in rats. J Comp Physiol A 192:497–505
Corcoran AE, Hodges MR, Wub Y, Wang W, Wylie CJ, Denerisc ES, Richerson GB (2009) Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol 168:49–58
de Carvalho D, Bícego KC, de Castro OW, da Silva GS, Garcia-Cairasco N, Gargaglioni LH (2010) Role of Neurokinin-1 expressing neurons in the Locus coeruleus on ventilatory and cardiovascular responses to hypercapnia. Respir Physiol Neurobiol 172:24–31
Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE (1990) Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience 36:207–216
Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG (2011) Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci 31:1981–1990
Dias MB, Li A, Nattie E (2008) Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal dialysis in the retrotrapezoid nucleus. J Appl Physiol 105:83–90
Dias MB, Nucci TB, Margatho LO, Antunes-Rodrigues J, Gargaglioni LH, Branco LG (2007) Raphe magnus nucleus is involved in ventilatory but not hypothermic response to CO2. J Appl Physiol 103:1780–1788
Di Pasquale E, Monteau R, Hilaire G (1994) Endogenous serotonin modulates the fetal respiratory rhythm: an in vitro study in the rat. Brain Res Dev Brain Res 80:222–232
Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, Martino P (2000) Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol 119:199–208
Forster HV, Smith CA (2010) Contributions of central and peripheral chemoreceptors to the ventilatory response to CO2/H+. J Appl Physiol 108:989–994
Gargaglioni LH, Coimbra N, Branco LGS (2003) The nucleus raphe magnus modulates hypoxia-induced hyperventilation but not anapyrexia in rats. Neurosci Lett 347:121–125
Hartzler LK, Dean JB, Putnam RW (2008) The chemosensitive response of neurons from the locus coeruleus (LC) to hypercapnic with clamped intracellular pH. Adv Exp Med Biol 605:333–337
Hilaire G, Voituron N, Menuet C, Ichiyama RM, Subramanian HH, Dutschmann M (2010) The role of serotonin in respiratory function and dysfunction. Respir Physiol Neurobiol 174:76–88
Hodges MR, Forster HV, Papanek PE, Dwinell MR, Hogan GE (2002) Ventilatory phenotypes among four strains of adult rats. J Appl Physiol 93:974–983
Hodges MR, Martino P, Davis S, Opansky C, Pan LG, Forster HV (2004) Effects on breathing of focal acidosis at multiple medullary raphe sites in awake goats. J Appl Physiol 97:2303–2309
Hodges MR, Opansky C, Qian B, Davis S, Bonis J, Bastasic J, Leekley T, Pan LG, Forster HV (2004) Transient attenuation of CO2 sensitivity after neurotoxic lesions in the medullary raphe area of awake goats. J Appl Physiol 97:2236–2247
Hodges MR, Richerson GB (2010) The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception and thermoregulation. J Appl Physiol 108:1425–1432
Hodges MR, Richerson GB (2010) Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir Physiol Neurobiol 173:256–263
Hodges MR, Tattersall GL, Harris MB, McEvoy S, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB (2008) Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28:2495–2505
Hodges MR, Opansky C, Qian B, Davis S, Bonis JM, Krause K, Pan LG, Forster HV (2005) Carotid body denervation alters ventilatory responses to ibotenic acid injections or focal acidosis in the medullary raphe. J Appl Physiol 98:1234–1242
Holtman JR, Anastasi NC, Dretchen KL (1986) Effect of electrical and chemical stimulation of the raphe obscurus on phrenic nerve activity in the cat. Brain Res 362:214–220
Li A, Nattie E (2002) CO2 dialysis in one chemoreceptor site, the RTN: stimulus intensity and sensitivity in the awake rat. Respir Physiol Neurobiol 133:11–22
Li A, Nattie E (2008) Serotonin transporter (5-HTT) knockout mice have a reduced ventilatory response to hypercapnia (predominantly in males) but not to hypoxia. J Physiol 586:2321–2329
Li A, Zhou S, Nattie E (2006) Simultaneous inhibition of caudal medullary raphe’ and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol (Lond) 577:307–318
Loeschcke HH, De Lattre J, Schlafke ME, Trouth CO (1970) Effects on respiration and circulation of electrically stimulating the ventral surface of the medulla oblongata. Respir Physiol 10:184–197
Loeschcke HH (1982) Central chemosensitivity and the reaction theory. J Physiol 332:1–24
Loeschcke HH (1973) Respiratory chemosensitivity in the medulla oblongata. Acta Neurobiol Exp 33:97–112
Malan A (1973) Ventilation measured by body plethysmography in hibernating mammals and in poikilotherms. Respir Physiol 17:32–44
Mauad H, Glass ML, Machado BH (1992) Effect of selective denervation of baroreceptors on pulmonary ventilation and arterial pressure lability in rat. Hypertension 19:182–186
Mitchell RA, Loeschcke HH, Massion WH, Severinghaus JW (1963) Respiratory responses mediated through superficial chemosensitive areas on the medulla. J Appl Physiol 18:523–533
Mitchell RA, Loeschcke HH, Severinghaus JW, Richardson BW, Massion WH (1963) Regions of respiratory chemosensitivity on the surface of the medulla. Ann NY Acad Sci 109:661–681
Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG (2004) Respiratory control by ventral surface chemoreceptor neurones in rats. Nat Neurosci 7:1360–1369
Mulkey DK, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG (2007) Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci 27:14128–14138
Nattie E (1999) CO2, brainstem chemoreceptors and breathing. Prog Neurobiol 59:299–331
Nattie E, Li A (2009) Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol 106:1464–1466
Nattie E, Li A (2001) CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol 90:1247–1257
Nattie E, Li A (2008) Multiple central chemoreceptor sites: cell types and function in vivo. Adv Exp Med Biol 605:343–347
Nattie E, Li A (2006) Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol 101:1596–1606
Nattie E (2000) Multiple sites for central chemoreception: their roles in response sensitivity and in sleep and wakefulness. Respir Physiol 122:223–235
Nattie EE, Li A, Richerson G, Lappi DA (2004) Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol 556:235–253
Nattie EE (2001) Central chemosensitivity, sleep, and wakefulness. Respir Physiol 129:257–268
Nichols NL, Mulkey DK, Wilkinson KA, Powell FL, Dean JB, Putnam RW (2009) Characterization of the chemosensitive response of individual solitary complex neurons from adult rats. Am J Physiol Regul Integr Comp Physiol 296:R763–R773
Nuding SC, Segers LS, Shannon R, O’Connor R, Morris KF, Lindsey BG (2009) Central and peripheral chemoreceptors evoke distinct responses in simultaneously recorded neurons of the raphe-pontomedullary respiratory network. Philos Trans R Soc Lond B Biol Sci 364:2501–2516
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 3rd edn. Academic, San Diego
Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC (2009) Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29:3720–3737
Putnam RW, Filosa JA, Ritucci NA (2004) Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287:C1493–C1526
Richerson GB (1995) Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol 73:933–944
Richerson GB (2004) Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5:449–461
Richerson GB, Wang W, Tiwari J, Bradley SR (2001) Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol 129:175–189
Schlaefke ME, See WR, Loeschcke HH (1970) Ventilatory response to alterations of H+-ion concentration in small areas of the ventral medullary surface. Respir Physiol 10:198–212
Serra A, Brozoski D, Hedin N, Franciosi R, Forster HV (2001) Mortality after carotid body denervation in rats. J Appl Physiol 3:1298–1306
Smith CA, Forster HV, Blain GM, Dempsey JA (2010) An interdependent model of central/peripheral chemoreception: evidence and implications for ventilatory control. Respir Physiol Neurobiol 173:288–297
Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA (2006) Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol 100:13–19
Stornetta RL, Moreira TS, Takakura AC, Bong Jin Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG (2006) Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26:10305–10314
Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG (2006) Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572:503–523
Taylor NC, Li A, Green A, Kinney HC, Nattie EE (2004) Chronic fluoxetine microdialysis into the medullary raphe nuclei of the rat, but not systemic administration, increases the ventilatory response to CO2. J Appl Physiol 97:1763–1773
Taylor NC, Li A, Nattie EE (2005) Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol 566:543–557
Veasey SC, Fornal CA, Metzler CW, Jacobs BL (1995) Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci 15:5346–5359
Zanella S, Watrin F, Mebarek S, Marly F, Roussel M, Gire C, Diene G, Tauber M, Muscatelli F, Hilaire G (2008) Necdin plays a role in the serotonergic modulation of the mouse respiratory network: implication for Prader–Willi syndrome. J Neurosci 28:1745–1755
Acknowledgements
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) no. 07/51581-2. Glauber S.F. da Silva was supported by FAPESP no. 06/60696-5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, G.S.F., Giusti, H., Benedetti, M. et al. Serotonergic neurons in the nucleus raphe obscurus contribute to interaction between central and peripheral ventilatory responses to hypercapnia. Pflugers Arch - Eur J Physiol 462, 407–418 (2011). https://doi.org/10.1007/s00424-011-0990-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-011-0990-x