Abstract
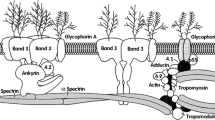
Previously, we reported that in mammalian erythrocytes irreversible annealing of spectrin heterodimers at 49–50 °C abolished cell volume-dependent regulation of ion carriers, thus suggesting an implication of a two-dimensional (2D) membrane carcass in volume sensing and/or signal transduction. To further examine this hypothesis, we employed atomic force microscopy. This method revealed folded membrane relief of fixed human erythrocytes with an average wave height of 3–5 nm covered by globular structures with a diameter of 40–50 nm and an average height of 1–2 nm. Erythrocyte swelling caused by reduction of medium osmolality decreased the height of membrane surface waves by 40 % and increased K+,Cl− cotransport by approximately sixfold. Both volume-sensitive changes of membrane relief and activity of K+,Cl− cotransporter were abolished by a 10-min preincubation at 50 °C. Our results strongly suggest that volume-dependent alterations of the human erythrocyte membrane relief are caused by reorganization of the 2D spectrin–actin network contributing to regulation of the activity of volume-sensitive ion transporters.





Similar content being viewed by others
References
Adragna N, Di Fulvio M, Lauf PK (2004) Regulation of K-Cl cotransport: from function to genes. J Membr Biol 201:109–137
Anfinogenova YJ, Rodriguez X, Grygorczyk R, Adragna N, Lauf PK, Hamet P, Orlov SN (2001) Swelling-induced K+ fluxes in vascular smooth muscle cells are mediated by charybdotoxin-sensitive K+ channels. Cell Physiol Biochem 11:295–310
Brandts JF, Taverna RD, Salasivan E, Lysko KA (1978) Calorimetric studies of structural transitions of human erythrocyte membrane. Studies of B and C transitions. Biochim Biophys Acta 512:566–578
Burton NM, Bruce LJ (2011) Modeling the structure of the red cell membrane. Biochem Cell Biol 89:200–215
Cheng Y, Liu M, Li R, Wang C, Bai C, Wang K (1999) Gadolinium induces domain and pore formation of human erythrocyte membrane: an atomic force microscopic study. Biochim Biophys Acta 1421:249–260
Evans J, Gratzer W, Mohandas N, Parker K, Sleep J (2008) Fluctuations of the red blood cell membrane: relation to mechanical properties and lack of ATP-dependence. Biophys J 94:4134–4144
Francis LW, Lewis PD, Wright CJ, Conlan RS (2010) Atomic force microscopy comes of age. Biol Cell 102:133–143
Garay R, Nazaret C, Hannaert R, Cragoe E (1988) Demonstration of a K+, Cl− cotransport system in human red blood cells by its sensitivity to [(dihydroindenyl)oxy]alcanoic acid regulation of cell swelling and distinction from bumetanide-sensitive Na+, K+, Cl− cotransport system. Mol Pharmacol 33:696–701
Girasole M, Pompeo G, Cricenti A, Congiu-Castellano A, Andreola F, Serafino A, Frazer BH, Boumis G, Amiconi G (2007) Roughness of the plasma membrane as an independent morphological parameter to study RBCs: A quantitative atomic force microscopy investigation. Biochim Biophys Acta 1768:1268–1276
Hoffmann EK, Lambert IH, Pedersen SF (2009) Physiology of cell volume regulation in vertebrates. Physiol Rev 89:193–277
Janmey PA, Lindberg U (2004) Cytoskeletal regulation: rich in lipids. Nat Rev Mol Cell Biol 5:658–666
Lang F, Busch G, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D (1998) Functional significance of cell volume regulatory mechanisms. Physiol Rev 78:247–306
Lauf PK, Adragna NC (2000) K-Cl cotransport: properties and molecular mechanism. Cell Physiol Biochem 10:341–354
Mongin AA, Orlov SN (2001) Mechanisms of cell volume regulation and possible nature of the cell volume sensor. Pathophysiology 8:77–88
Nasuhoglu C, Feng S, Mao Y, Shammat I, Yamamato M, Earnest S, Lemmon M, Hilgemann DW (2002) Modulation of cardiac PIP2 by cardioactive hormones and other physiologically relevant interventions. Am J Physiol Cell Physiol 283:C223–C234
Nielsen DK, Jensen AK, Harbak H, Christensen SC, Simonsen LO (2007) Cell content of phosphatidylinositol (4,5)biphosphate in Ehrlich mouse ascites tumour cells in response to cell volume perturbations in anisotonic and isosmotic media. J Physiol 582:1027–1036
Oberleithner H (2008) Nanophysiology: fact or fiction? Pfluger Arch.- Eur. J Physiol 456:1–2
Ohta Y, Otsuka C, Okamoto H (2003) Changes in surface roughness of erythrocytes due to shear stress: atomic force microscopic visualization of the surface microstructure. J Artif Organs 6:101–105
O’Neill WC (1999) Physiological significance of volume-regulated transporters. Am J Physiol 276:C995–C1011
Orlov SN, Kolosova IA, Cragoe EJ, Gurlo TG, Mongin AA, Aksentsev SL, Konev SV (1993) Kinetics and peculiarities of thermal inactivation of volume-dependent Na/H exchange, Na, K,2Cl cotransport and K, Cl cotransport in rat erythrocytes. Biochim Biophys Acta 1151:186–192
Orlov SN, Kuznetsov SR, Kolosova IA, Aksentsev SL, Konev SV (1997) Volume-dependent regulation of ion transporters in human and rat erythrocytes: role of cytoskeleton and protein phosphorylation. Russ Physiol J 83(No5–6):119–147
Orlov SN, Pokudin NI, Kotelevtsev YV, Gulak PV (1989) Volume-dependent regulation of ion transport and membrane phosphorylation in human and rat erythrocytes. J Membr Biol 107:105–117
Park Y, Best CA, Auth T, Gov NS, Safran SA, Popescu G, Suresh S, Feld MS (2010) Metabolic remodelling of the human red blood cell membrane. Proc Natl Acad Sci USA 107:1289–1294
Repin NV, Bobrova EN, Repina SV (2008) Thermally induced transformation of mammalian red blood cells during hyperthermia. Bioelectrochemistry 73:101–105
Sachs JR (1998) How do red blood cells know how big they are? In: Okada Y (ed) Cell volume regulation: the molecular mechanism and volume sensing machinery. Elsevier Science, Tokyo, pp 3–13
Shnyrov VL, Orlov SN, Zhadan GG, Pokudin NI (1990) Thermal inactivation of membrane proteins, volume-dependent Na+, K+ cotransport, and protein kinase C activator-induced changes of the shape of human and rat erythrocytes. Biomed Biochim Acta 49:445–453
Strange K (2004) Cellular volume homeostasis. Adv Physiol Educ 28:155–159
Tuvia S, Levin S, Bitler A, Korenstein R (1998) Mechanical fluctuation of the membrane-skeleton are dependent on F-actin ATPase in human erythrocytes. J Cell Biol 141:1551–1561
Yamamoto M, Chen MZ, Wang YJ, Sun HQ, Wei Y, Martinez M, Yin HL (2006) Hypertonic stress increases phosphatidylinositol 4,5-biphosphate levels by activating PIP5KIbeta. J Biol Chem 281:32630–32638
Yusipovich AI, Zagubizhenko MV, Levin GG, Platonova A, Parshina EY, Grygorczyk R, Maksimov GV, Rubin AB, Orlov SN (2011) Laser interference microscopy of amphibian erythrocytes: impact of cell volume and refractive index. J Microsc 244:223–229
Acknowledgments
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (to R.G. and S.N.O.), the Russian Foundation for Fundamental Research (12-04-00033-a to S.N.O; 10-04-00835-а to G.V.M. and E.Y.P.), and the Ministry of Education and Science of the Russian Federation (projects ##8477 and 8162). Manuscript editing by Ovid Da Silva and logistical services from the Research Support Office, CRCHUM are appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parshina, E.Y., Yusipovich, A.I., Platonova, A.A. et al. Thermal inactivation of volume-sensitive K+,Cl− cotransport and plasma membrane relief changes in human erythrocytes. Pflugers Arch - Eur J Physiol 465, 977–983 (2013). https://doi.org/10.1007/s00424-013-1221-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1221-4