Abstract
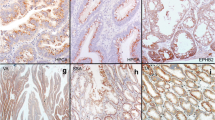
Serrated adenocarcinoma (SAC), representing at least 10 % of colorectal carcinomas (CRC), differs from conventional carcinomas not only by its histology, but also by its molecular basis. However, the diagnosis of SAC in poorly differentiated cases and without an adjacent serrated adenoma can be challenging. In this study, we utilized previously described expression data and identified annexin A10 (ANXA10) as a potential marker for SAC. We conducted ANXA10 immunohistochemistry in groups of 146 CRC patients and 131 serrated and conventional polyps. In CRC cases, ANXA10 expression associated with serrated histology (sensitivity 42 % and specificity 98 %). BRAF V600E mutation correlated with ANXA10 expression but also seven BRAF wild-type tumors (5 %) were positive for ANXA10. Immunoreactivity for either ANXA10 or BRAF V600E was an accurate predictor of serrated histology (sensitivity 55 % and specificity 97 %). ANXA10 expression did not associate with tumor stage or grade. Of the 131 colorectal polyps, 30/30 of sessile serrated adenomas, 6/11 traditional serrated adenomas, 20/32 hyperplastic polyps, and 2/27 tubulovillous adenomas were positive for ANXA10, while 31/31 tubular adenomas were negative. In conclusion, the results suggest that ANXA10 is a marker with high specificity for the serrated pathway of CRC.


Similar content being viewed by others
Abbreviations
- ANXA10:
-
Annexin A10
- CC:
-
Conventional adenocarcinoma
- CRC:
-
Colorectal cancer
- GCHP:
-
Goblet cell rich hyperplastic polyp
- HP:
-
Hyperplastic polyp
- IHC:
-
Immunohistochemistry
- MAPK-ERK:
-
Mitogen-activated protein kinase-extracellular signal-regulated kinase
- MMR:
-
Mismatch repair
- MSI:
-
Microsatellite instability
- MVHP:
-
Microvesicular hyperplastic polyp
- SAC:
-
Serrated adenocarcinoma
- SSA:
-
Sessile serrated adenoma
- TMA:
-
Tissue microarray
- TSA:
-
Traditional serrated adenoma
References
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer Statistics, 2014. CA Cancer J Clin 64:9–29. doi:10.3322/caac.21208
O’Brien MJ, Yang S, Huang CS et al (2008) The serrated polyp pathway to colorectal carcinoma. Diagn Histopathol 14:78–93. doi:10.1016/j.mpdhp.2007.12.003
Mäkinen MJ, George SM, Jernvall P et al (2001) Colorectal carcinoma associated with serrated adenoma—Prevalence, histological features, and prognosis. J Pathol 193:286–294. doi:10.1002/1096-9896(2000)9999:9999<::AID-PATH800>3.0.CO;2-2
Makinen MJ (2007) Colorectal serrated adenocarcinoma. Histopathology 50:131–150. doi:10.1111/j.1365-2559.2006.02548.x
Jass JR (2007) Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 50:113–130. doi:10.1111/j.1365-2559.2006.02549.x
Tuppurainen K, Makinen JM, Junttila O et al (2005) Morphology and microsatellite instability in sporadic serrated and non-serrated colorectal cancer. J Pathol 207:285–294. doi:10.1002/path.1850
Snover D, Ahnen D, Burt R, Odze R (2010) Serrated polyps of the colon and rectum and serrated (“hyperplastic”) polyposis. WHO Classif. tumours. Pathol. Genet. Tumours Dig. Syst. Berlin Springer-Verlag (4th Ed)
García-Solano J, Pérez-Guillermo M, Conesa-Zamora P et al (2010) Clinicopathologic study of 85 colorectal serrated adenocarcinomas: Further insights into the full recognition of a new subset of colorectal carcinoma. Hum Pathol 41:1359–1368. doi:10.1016/j.humpath.2010.04.002
Kambara T, Simms LA, Whitehall VLJ et al (2004) BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut 53:1137–1144. doi:10.1136/gut.2003.037671
O’Brien MJ, Yang S, Mack C et al (2006) Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 30:1491–1501. doi:10.1097/01.pas.0000213313.36306.85
McGivern A, Wynter CV, Whitehall VL et al (2004) Promoter hypermethylation frequency and BRAF mutations distinguish hereditary non-polyposis colon cancer from sporadic MSI-H colon cancer. Fam Cancer 3:101–107
Poynter JN, Siegmund KD, Weisenberger DJ et al (2008) Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev 17:3208–3215. doi:10.1158/1055-9965.EPI-08-0512
Spring KJ, Zhao ZZ, Karamatic R et al (2006) High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 131:1400–1407. doi:10.1053/j.gastro.2006.08.038
Burnett-Hartman AN, Newcomb PA, Potter JD et al (2013) Genomic aberrations occurring in subsets of serrated colorectal lesions but not conventional adenomas. Cancer Res 73:2863–2872
Stefanius K, Ylitalo L, Tuomisto A et al (2011) Frequent mutations of KRAS in addition to BRAF in colorectal serrated adenocarcinoma. Histopathology 58:679–692. doi:10.1111/j.1365-2559.2011.03821.x
Sajanti SA, Sirniö P, Väyrynen JP et al (2014) VE1 immunohistochemistry accurately detects BRAF V600E mutations in colorectal carcinoma and can be utilized in the detection of poorly differentiated colorectal serrated adenocarcinoma. Virchows Arch. doi:10.1007/s00428-014-1555-0
Laiho P, Kokko A, Vanharanta S et al (2007) Serrated carcinomas form a subclass of colorectal cancer with distinct molecular basis. Oncogene 26:312–320. doi:10.1038/sj.onc.1209778
Conesa-Zamora P, García-Solano J, García-García F et al (2013) Expression profiling shows differential molecular pathways and provides potential new diagnostic biomarkers for colorectal serrated adenocarcinoma. Int J Cancer 132:297–307. doi:10.1002/ijc.27674
Mussunoor S, Murray G (2008) The role of annexins in tumour development and progression. J Pathol 216:131–140. doi:10.1002/path
Kantola T, Klintrup K, Väyrynen JP et al (2012) Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer 107:1729–1736. doi:10.1038/bjc.2012.456
Hamilton SR, Bosman FT, Boffetta P et al (2010) Carcinoma of the colon and rectum. In: Bosman F, Carneiro F, Hruban R, Theise N (eds) WHO Classif. tumours Dig. Syst. IARC Press, Lyon, pp 134–146
Sobin LH, Wittekind C (2002) TNM classification of malignant tumours. Wiley-Liss, New York
Väyrynen JP, Vornanen J, Tervahartiala T et al (2012) Serum MMP-8 levels increase in colorectal cancer and correlate with disease course and inflammatory properties of primary tumors. Int J Cancer 131:E463–E474. doi:10.1002/ijc.26435
Väyrynen JP, Sajanti SA, Klintrup K et al (2014) Characteristics and significance of colorectal cancer associated lymphoid reaction. Int J Cancer 134:2126–2135. doi:10.1002/ijc.28533
Gerke V, Moss SE (2002) Annexins: from structure to function. Physiol Rev 82:331–371. doi:10.1152/physrev.00030.2001
Duncan R, Carpenter B, Main LC et al (2008) Characterisation and protein expression profiling of annexins in colorectal cancer. Br J Cancer 98:426–433. doi:10.1038/sj.bjc.6604128
Quiskamp N, Poeter M, Raabe CA et al (2014) The tumor suppressor annexin A10 is a novel component of nuclear paraspeckles. Cell Mol Life Sci 71:311–329. doi:10.1007/s00018-013-1375-4
Lu S-H, Yuan R-H, Chen Y-L et al (2013) Annexin A10 is an immunohistochemical marker for adenocarcinoma of the upper gastrointestinal tract and pancreatobiliary system. Histopathology 63:640–648. doi:10.1111/his.12229
Kim J, Kim MA, Jee CD et al (2009) Reduced expression and homozygous deletion of annexin A10 in gastric carcinoma. Int J Cancer 125:1842–1850. doi:10.1002/ijc.24541
Kim JKYU, Kim PUMJ, Jung KHWA et al (2010) Decreased expression of Annexin A10 in gastric cancer and its overexpression in tumor cell growth suppression. Oncol Rep 24:607–612. doi:10.3892/or
Munksgaard PP, Mansilla F, Brems Eskildsen A-S et al (2011) Low ANXA10 expression is associated with disease aggressiveness in bladder cancer. Br J Cancer 105:1379–1387. doi:10.1038/bjc.2011.404
Liu S-H, Lin C-Y, Peng S-Y et al (2002) Down-regulation of annexin A10 in hepatocellular carcinoma is associated with vascular invasion, early recurrence, and poor prognosis in synergy with p53 mutation. Am J Pathol 160:1831–1837. doi:10.1016/S0002-9440(10)61129-7
Patsos G, Germann A, Gebert J, Dihlmann S (2010) Restoration of absent in melanoma 2 (AIM2) induces G2/M cell cycle arrest and promotes invasion of colorectal cancer cells. Int J Cancer 126:1838–1849. doi:10.1002/ijc.24905
Gonzalo DH, Lai KK, Shadrach B et al (2013) Gene expression profiling of serrated polyps identifies annexin A10 as a marker of a sessile serrated adenoma/polyp. J Pathol 230:420–429. doi:10.1002/path.4200
Dhillon AS, Hagan S, Rath O, Kolch W (2007) MAP kinase signalling pathways in cancer. Oncogene 26:3279–3290. doi:10.1038/sj.onc.1210421
Fearon ER (2011) Molecular genetics of colorectal cancer. Annu Rev Pathol 6:479–507. doi:10.1146/annurev-pathol-011110-130235
Shimizu T, Kasamatsu A, Yamamoto A et al (2012) Annexin A10 in human oral cancer: Biomarker for tumoral growth via G1/S transition by targeting MAPK signaling pathways. PLoS One 7:e45510. doi:10.1371/journal.pone.0045510
Haigis KM, Kendall KR, Wang Y et al (2008) Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet 40:600–608. doi:10.1038/ng.115
Rad R, Cadiñanos J, Rad L et al (2013) A genetic progression model of Braf(V600E)-induced intestinal tumorigenesis reveals targets for therapeutic intervention. Cancer Cell 24:15–29. doi:10.1016/j.ccr.2013.05.014
Yeh JJ, Routh ED, Rubinas T et al (2009) KRAS/BRAF mutation status and ERK1/2 activation as biomarkers for MEK1/2 inhibitor therapy in colorectal cancer. Mol Cancer Ther 8:834–843. doi:10.1158/1535-7163.MCT-08-0972
Acknowledgments
The authors wish to express their gratitude to Ms Riitta Vuento for her excellent assistance in the preparation of study material. This study was supported by grants from the Academy of Finland, Emil Aaltonen Foundation, Finnish Cancer Society, Finnish Foundation for Gastroenterological Research, Finnish Medical Foundation, Northern Finland Cancer Foundation, and Orion-Farmos Research Foundation, Oulu University Scholarship Foundation.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sajanti, S.A., Väyrynen, J.P., Sirniö, P. et al. Annexin A10 is a marker for the serrated pathway of colorectal carcinoma. Virchows Arch 466, 5–12 (2015). https://doi.org/10.1007/s00428-014-1683-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1683-6