Abstract
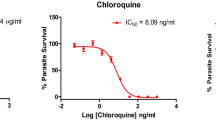
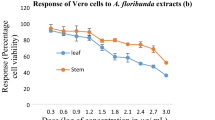
In vitro antiplasmodial activity of methanolic extracts of 16 medicinal plants was evaluated by fluorometric assay using PicoGreen. The IC50s, as determined by parasite DNA concentration, ranged from <11 to >200 and <13 to >200 μg/ml for Plasmodium falciparum 3D7 and K1, respectively; and the most active extracts were those from Anogeissus leiocarpus and Terminalia avicennoides (<11–≥14 μg/ml). Aqueous, butanolic, ethyl acetate, and methanolic fractions of these two extracts revealed butanolic fraction to have a relatively better activity (IC50, 10–12 μg/ml). Activity-guided chromatographic separation of the butanolic fraction on Sephadex LH-20 followed by nuclear magnetic resonance and correlation high-performance liquid chromatography revealed the presence of known hydrolysable tannins and some related compounds—castalagin, ellagic acid, flavogallonic acid, punicalagin, terchebulin, and two other fractions. The IC50s of all these compounds ranged between 8–21 μg/ml (8–40 μM) against both the strains. Toxicity assay with mouse fibroblasts showed all the extracts and isolated compounds to have IC50 ≥ 1500 μg/ml, except for Momordica balsamina with <1500 μg/l. All the extracts and isolated compounds did not affect the integrity of human erythrocyte membrane at the observed IC50s. However, adverse effects manifest in a concentration-dependent fashion (from IC50 ≥ 500 μg/ml).




Similar content being viewed by others
References
Abdullahi AL, Agho MO, Amos S, Gamaniel KS, Wambebe C (2001) Antidiarrhoeal activity of the aqueous extract of Terminalia avicennoides roots. Phytother Res 15:431–434
Adamu HM, Abayeh OJ, Agho MO, Abdullahi AL, Uba A, Dukku HU, Wufem BM (2005) An ethnobotanical survey of Bauchi State herbal plants and their antimicrobial activity. J Ethnopharmacol 99:1–4
Akah PA, Orisakwe OE, Gamaniel KS, Shittu A (1998) Evaluation of Nigerian traditional medicines: II. Effects of some Nigerian folk remedies on peptic ulcer. J Ethnopharmacol 62:123–127
Asres K, Bucar F, Edelsbrunner S, Kartnig T, Hoger G, Thiel W (2001a) Investigations on antimycobacterial activity of some Ethiopian medicinal plants. Phytother Res 15:323–326
Asres K, Bucar F, Knauder E, Yardley V, Kendrick H, Croft SL (2001b) In vitro antiprotozoal activity of extract and compounds from the stem bark of Combretum Molle. Phytother Res 15:613–617
Bizimana N, Tietjen U, Zessin KH, Diallo D, Djibril C, Melzig MF, Clausen PH (2006) Evaluation of medicinal plants from Mali for their in vitro and in vivo trypanocidal activity. J Ethnopharmacol 103:350–356
Bruce-Chwatt LJ (1988) Cinchona and its alkaloids: 350 years. NY State J Med 88:318–322
Cailean C, Vinesh JM, Neil RC, Olwen MG, Pamisha P, Motlalepula GM, Niresh G, Peter JS, Peter IF (2004) In vitro antiplasmodial activity of medicinal plants native to or naturalized in South Africa. J Ethnopharmacol 92:177–191
Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S (2004) Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov 3:509–520
Fleming LW (1999) A medical bouquet. Poppies, cinchona and willow. Scottish Med J 44:176–179
Govindarajan R, Vijayakumar M, Singh M, Rao ChV, Shirwaikar A, Rawat AKS, Pushpangadan P (2006) Antiulcer and antimicrobial activity of Anogeissus latifolia. J Ethnopharmacol 106:57–61
Kamtchouing P, Kahpui SM, Djomeni Dzeufiet PD, Tedong LEA, Asongalem EA, Dimo T (2006) Anti-diabetic activity of methanol/methylene chloride stem bark extracts of Terminalia superba and Canarium schweinfurthii on streptozotocin-induced diabetic rats. J Ethnopharmacol 104:306–309
Larrosa M, Tomas-Barberan FA, Espın JC (2006) The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem 17:611–625
Lin T, Nonaka G, Nishioka I, Ho F (1990) Tannins and related compounds.CII. Structures of terchebulin, an ellagitannin having a novel tetraphenylcarboxylic acid (terchebulic acid) moiety, and biogenetically related tannins from Terminalia chebula RETZ. Chem Pharm Bull 38:3004–3008
Phillipson JD, Wright CW, Kirby GC, Warhurst DC (1993) Tropical plants as sources of antiprotozoal agents. Recent Adv Phytochem 27:1–40
Quideau S, Varadinova T, Karagiozova D, Jourdes M, Pardon M, Baudry C, Genova P, Diakov T, Petrova R (2004) Main structural and stereochemical aspects of the antiherpetic activity of nonahydroxyterphenoyl-containing C-glycosidic ellagitannins. Chem Biodivers 1:247–258
Rotimi VO, Laughon BE, Bartlett JG, Mosadomi HA (1988) Activities of Nigerian chewing stick extracts against Bacteroides gingivalis and Bacteroides melaninogenicus. Antimicrob Agents Chemother 32:598–600
Sirima SB, Gansane A (2007) Artesunate–amodiaquine for the treatment of uncomplicated malaria. Expert Opin Investig Drugs 16:1079–1085
Taguri T, Tanaka T, Kouno I (2004) Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol Pharm Bull 27:1965–1969
Taiwo O, Xu H-X, Lee SF (1999) Antibacterial activities of extracts from Nigerian chewing sticks. Phytother Res 13:675–679
Tanaka T, Nonaka G, Nishioka I (1986a) Tannins and related compounds. XLI. Isolation and characterization of novel ellagitannins, punicacorteins A, B, C and D, and punigluconin from the bark of Punica granatum L. Chem Pharm Bull 34:656–663
Tanaka T, Nonaka G, Nishioka I (1986b) Tannins and related compounds. XLII. Isolation and characterization of four new hydrolyzable tannins, terflavins A and B, tergallagin and tercatain from the leaves of Terminalia catappa L. Chem Pharm Bull 4:1039–1049
Tanaka T, Ueda N, Shinohara H, Nonaka G, Fujioka T, Mihashi K, Kouno I (1996) C-glycosidic ellagitannin metabolites in the heartwood of Japanese chestnut tree (Castanea crenata Sieb. et Zucc.). Chem Pharm Bull 44:2236–2242
Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193:673–675
Vonthron-Sénécheau C, Weniger B, Ouattara M, Tra Bi F, Kamenan A, Lobstein A, Brun R, Anton R (2003) In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected Ivorian plants. J Ethnopharmacol 87:221–225
WHO (2003) Fact sheet N°134, Revised May, 2003. Traditional medicine
WHO (2007) www.who.int/mediacentre/factsheets/fs094/en/index.html
Woerdenbag HJ, Lugt CB, Pras N (1990) Artemisia annua L.: a source of novel antimalarial drugs. Pharm Weekbl Sci 12:169–181
Yang LL, Lee CY, Yen KY (2000) Induction of apoptosis by hydrolyzable tannins from Eugenia jambos L. on human leukemia cells. Cancer Lett 157:65–75
Yolanda C, Liuris H, Jose G, Luis C, Todd LC, Phyllis DC, Thomas AK, Luzi R, Eduardo O-B (2004) A novel DNA-based microfluorometric method to evaluate antimalarial drug activity. Am J Trop Med Hyg 70:119–124
Ziegler HL, Franzyk H, Sairafianpour M, Tabatabai M, Tehrani MD, Bagherzadeh K, Hagerstrand H, Staerk D, Jaroszewski JW (2004) Erythrocyte membrane modifying agents and the inhibition of Plasmodium falciparum growth: structure–activity relationships for betulinic acid analogues. Bioorg Med Chem 12:119–127
Acknowledgements
This work was supported by Japanese Society for the Promotion of Science (JSPS). MNS is a JSPS fellow (ID#: 05166) and wish to thank, Dr. Haruna Danwanka, Mallam Farouq and Sabo Miri of Abuabakar Tafawa Balewa University, Bauchi, Nigeria; for their cooperation during the crude extract preparations and Dr. Toshihide Mitamura of Research Institute International Medical Center of Japan, Tokyo for the supply of the P. falciparum strains and his useful comment on the work and manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shuaibu, M.N., Wuyep, P.A., Yanagi, T. et al. The use of microfluorometric method for activity-guided isolation of antiplasmodial compound from plant extracts. Parasitol Res 102, 1119–1127 (2008). https://doi.org/10.1007/s00436-008-0879-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-0879-6