Abstract
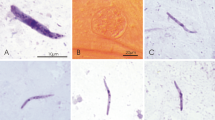
Haemoproteus spp. are cosmopolitan vector-born haemosporidian parasites, some species of which cause diseases in non-adapted birds. Recent polymerase chain reaction (PCR)-based studies have detected mitochondrial cytochrome b gene lineages of these Haemoproteus parasites in blood-sucking mosquitoes and speculated about possible involvement of these insects in transmission of avian haemoproteids. However, development of Haemoproteus lineages has not been documented in mosquitoes. We infected 304 individuals of Ochlerotatus cantans, a widespread Eurasian mosquito, with Haemoproteus tartakovskyi (lineage hSISKIN1) and Haemoproteus balmorali (lineage hROBIN1). Mosquitoes were allowed to take non-infected and infected blood meals and maintained in the laboratory until 17 days post-infection (dpi). They were tested for presence of sporogonic stages by microscopic and PCR-based methods. Microscopic examination revealed partial development of both parasites in the infected insects. Numerous ookinetes were seen in the gut area and adjacent tissues located in the head, thorax and abdomen of mosquitoes between 1 and 5 dpi. Numerous oocysts were seen in the midgut wall between 4 and 15 dpi; they were also present in the head and thorax of infected mosquitoes testifying to the active movement of ookinetes throughout the body. Oocysts degenerated between 11 and 17 dpi. Sporozoites were not seen in oocysts or mosquito salivary glands, indicating abortive sporogonic development at the oocyst stage. In accordance with microscopy data, PCR and sequencing revealed presence of the lineages hSISKIN1 and hROBIN1 in experimental mosquitoes as long as 15 and 17 dpi, respectively, demonstrating relatively long survival of Haemoproteus parasites in the resistant insects without DNA degeneration. The present study shows that PCR-based diagnostics should be carefully used in vector studies of haemosporidians because it detects parasites in insects for several weeks after initial infection, but does not distinguish abortive parasite development. Demonstration of infective sporozoites in insects is essential for definitively demonstrating the insects are vectors.


Similar content being viewed by others
References
Arai M, Billker O, Morris HR, Panico M, Delcroix M, Dixon D, Ley SV, Sinden RE (2001) Both mosquito-derived xanthurenic acid and a host blood-derived factor regulate gametogenesis of Plasmodium in the midgut of the mosquito. Mol Biochem Parasit 116:17–24. doi:10.1016/S0166-6851(01)00299-7
Arez AP, Lopes D, Pinto J, Franco AS, Snounou G, do Rosario VE (2000) Plasmodium spp.: optimal protocols for PCR detection of low parasite numbers from mosquito (Anopheles spp.) samples. Exp Parasitol 94:269–272
Atkinson CT, van Riper CIII (1991) Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loye JE, Zuk M (eds) Bird–parasite interactions: ecology, evolution, and behaviour. Oxford University Press, Oxford, pp 19–48
Atkinson CT, Forrester DJ, Greiner EC (1988) Pathogenicity of Haemoproteus meleagridis (Haemosporina: Haemoproteidae) in experimentally infected domestic turkeys. J Parasitol 74:228–239
Bairlein F (1986) Ein standardisiertes Futter für Ernährungsuntersuchungen an omnivoren Kleinvögeln. J Ornithol 127:338–340
Baker JR (1957) A new vector of Haemoproteus columbae in England. J Protozool 4:204–208
Baker JR (1966) Haemoproteus palumbis sp. nov. (Sporozoa, Haemosporina) of the English wood-pigeon Columba p. palumbus. J Protozool 13:515–519
Beier JC (1998) Malaria parasite development in mosquitoes. Annu Rev Entomol 43:519–543
Bennett GF, Garnham PCC, Fallis AM (1965) On the status of the genera Leucocytozoon Ziemann, 1898 and Haemoproteus Kruse, 1890 (Haemosporidia: Leucocytozoidae and Haemoproteidae). Can J Zool 43:927–932
Bennett GF, Earlé RA, Du Toit H, Huchzermeyer FW (1992) A host–parasite catalogue of the haematozoa of the sub-Saharan birds. Onderstepoort J Vet Res 59:1–73
Bennett GF, Peirce MA, Ashford RW (1993) Avian haematozoa: mortality and pathogenicity. J Nat Hist 27:993–1001
Bensch S, Stjenman M, Hasselquist D, Östman Ö, Hansson B, Westerdahl H, Torres-Pinheiro R (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc R Soc B 276:1583–1589
Bensch S, Hellgren O, Pérez-Tris J (2009) A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358. doi:10.1111/j.1755-0998.2009.02692.x
Bernotienė R (2012) The fauna and seasonal activity of mosquitoes (Diptera: Culicidae) in the Curonian Spit (Russia, Lithuania). Euro Mosq Bull 30:72–78
Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, Rogers M, Sinden RE, Morris HR (1998) Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 392:289–292
Bishop MA, Bennett GF (1992) Host–parasite catalogue of the avian haematozoa: supplement 1, and bibliography of the avian blood-inhabiting haematozoa: supplement 2. Meml Univ Nfld Occas Pap Biol 15:1–244
Cardona CJ, Ihejirika A, McClellan L (2002) Haemoproteus lophortyx infection in bobwhite quail. Avian Dis 46:249–255
Carlson JS, Martínez-Gómez JE, Cornel A, Loiseau C, Sehgal RN (2011) Implications of Plasmodium parasite infected mosquitoes on an insular avifauna: the case of Socorro Island, México. J Vector Ecol 36:213–320. doi:10.1111/j.1948-7134.2011.00159.x
Desser SS, Bennett GF (1993) The genera Leucocytozoon, Haemoproteus and Hepatocystis. In: Kreier JP, Baker JR (eds) Parasitic protozoa, vol 4, 2nd edn. Academic, New York, pp 273–307
Donovan TA, Schrenzel M, Tucker TA, Pessier AP, Stalis IH (2008) Hepatic hemorrhage, hemocoelom, and sudden death due to Haemoproteus infection in passerine birds: eleven cases. J Vet Diagn Invest 20:304–313
Ejiri H, Sato Y, Sawai R, Sasaki E, Matsumoto R, Ueda M, Higa Y, Tsuda Y, Omori S, Murata K, Yukawa M (2009) Prevalence of avian malaria parasite in mosquitoes collected at a zoological garden in Japan. Parasitol Res 105:629–633. doi:10.1007/s00436-009-1434-9
Ejiri H, Sato Y, Kim KS, Tsuda Y, Murata K, Saito K, Watanabe Y, Shimura Y, Yukawa M (2011) Blood meal identification and prevalence of avian malaria parasite in mosquitoes collected at Kushiro Wetland, a subarctic zone of Japan. J Med Entomol 48:904–908
Fabian MM, Toma H, Arakawa T, Sato Y (2004) Malaria parasite developmental analyses by the nested polymerase chain reaction method: an implication for the evaluation of mosquito infection rates in epidemiological studies. Southeast Asian J Trop Med Public Health 35:820–827
Fallis AM, Bennett GF (1960) Description of Haemoproteus canachites n. sp. (Sporozoa: Haemoproteidae) and sporogony in Culicoides (Diptera: Ceratopogonidae). Can J Zool 38:455–464
Ferrell ST, Snowden K, Marlar AB, Garner M, Lung NP (2007) Fatal hemoprotozoal infections in multiple avian species in a zoological park. J Zoo Wildlife Med 38:309–316
Foley DH, Harrison G, Murphy JR, Dowler M, Rueda LM, Wilkerson RC (2012) Mosquito bisection as a variable in estimates of PCR-derived malaria sporozoites rates. Malar J 11:145. doi:10.1186/1475-2875-11-145
Garnham PCC (1966) Malaria parasites and other haemosporidia. Blackwell, Oxford
Glaizot O, Fumagalli L, Iritano K, Lalubin F, Van Rooyen J, Christe P (2012) High prevalence and lineage diversity of avian malaria in wild populations of great tits (Parus major) and mosquitoes (Culex pipiens). PLoS One 7:e34964. doi:10.1371/journal.pone.0034964
Greiner EC, Bennett GF, White EM, Coombs RF (1975) Distribution of the avian hematozoa of North America. Can J Zool 53:1762–1787
Hall TA (1999) BioEdit: a user—friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symp Ser (Oxf) 41:95–98
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol 90:797–802
Iezhova TA, Dodge M, Sehgal RN, Smith TB, Valkiūnas G (2011) New avian Haemoproteus species (Haemosporida: Haemoproteidae) from African birds, with a critique of the use of host taxonomic information in hemoproteid classification. J Parasit 97:682–694. doi:10.1645/GE-2709.1
Ishtiaq F, Guillaumot L, Clegg SM, Phillimore AB, Black RA, Owens IPF, Mundy NI, Sheldon BC (2008) Avian haematozoan parasites and their associations with mosquitoes across Southwest Pacific Islands. Mol Ecol 17:4545–4555. doi:10.1111/j.1365-294X.2008.03935.x
Kazlauskienė R, Bernotienė R, Palinauskas V, Iezhova TA, Valkiūnas G (2013) Plasmodium relictum (lineages pSGS1 and pGRW11): Complete synchronous sporogony in mosquitoes Culex pipiens pipiens. Exp Parasitol 133:454–461. doi:10.1016/j.exppara.2013.01.008
Kim KS, Tsuda Y (2012) Avian Plasmodium lineages found in spot surveys of mosquitoes from 2007 to 2010 at Sakata wetland, Japan: do dominant lineages persist for multiple years? Mol Ecol 21:5374–5385. doi:10.1111/mec.12047
Kim KS, Tsuda Y, Sasaki T, Kobayashi M, Hirota Y (2009) Mosquito blood–meal analysis for avian malaria study in wild bird communities: laboratory verification and application to Culex sasai (Diptera: Culicidae) collected in Tokyo, Japan. Parasitol Res 105:1351–1357. doi:10.1007/s00436-009-1568-9
Kimura M, Darbro JM, Harrington LC (2010) Avian malaria parasites share congeneric mosquito vectors. J Parasitol 96:144–151. doi:10.1645/GE-2060.1
Kocher TD, Thomas WK, Meyer A, Edwards SV, Paabo S, Villablanca FX, Wilson AC (1989) Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci USA 86:6196–6200
Križanauskienė A, Iezhova TA, Palinauskas V, Chernetsov N, Valkiūnas G (2012) Haemoproteus nucleocondensus n. sp. (Haemosporida, Haemoproteidae) from a Eurasian songbird, the Great Reed Warbler Acrocephalus arundinaceus. Zootaxa 3441:36–46
Levin II, Valkiūnas G, Iezhova TA, O'Brien SL, Parker PG (2012) Novel Haemoproteus species (Haemosporida: Haemoproteidae) from the swallow-tailed gull (Lariidae), with remarks on the host range of hippoboscid-transmitted avian hemoproteids. J Parasitol 98:847–854. doi:10.1645/GE-3007.1
Malmqvist B, Strasevičius D, Hellgren O, Adler PH, Bensch S (2004) Vertebrate host specificity of wild-caught blackflies revealed by mitochondrial DNA in blood. Proc Biol Sci 271:152–155
Martínez-de la Puente J, Martínez J, Rivero-de Aguilar J, Herrero J, Merino S (2011) On the specificity of avian blood parasites: revealing specific and generalist relationships between haemosporidians and biting midges. Mol Ecol 20:3275–3287. doi:10.1111/j.1365-294X.2011.05136.x
Martinsen ES, Paperna I, Schall JJ (2006) Morphological versus molecular identification of avian Haemosporidia: an exploration of three species concepts. Parasitology 133:279–288
Martinsen EM, Perkins SL, Schall JJ (2008) A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol 47:261–273. doi:10.1016/j.ympev.2007.11.012
Marzal A, de Lopes F, Navarro C, Møller AP (2005) Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142:541–545
McClure HE, Poonswad P, Greiner EC, Laird M (1978) Haematozoa in the birds of Eastern and Southern Asia. Memorial University of Newfoundland, St. John’s
Merino S, Moreno J, Sanz JJ, Arriero E (2000) Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). Proc Biol Sci 267:2507–2510
Miltgen F, Landau I, Ratanaworabhan N, Yenbutra S (1981) Parahaemoproteus desseri n. sp.; Gamétogonie et schizogonie chez l’hôte naturel: Psittacula roseata de Thailande, et sporogonie expérimentale chez Culicoides nubeculosus. Ann Parasitol Hum Comp 56:123–130
Møller AP, Nielsen JT (2007) Malaria and risk of predation: a comparative study of birds. Ecology 88:871–881
Nayar JK, Young MD, Forrester DJ (1980) Wyeomyia vanduzeei, an experimental host for wild turkey malaria Plasmodium hermani. J Parasitol 66:166–167
Njabo K, Cornel AJ, Sehgal RNM, Loiseau C, Buermann W, Harrigan RJ, Pollinger J, Valkiūnas G, Smith TB (2009) Coquillettidia (Culicidae, Diptera) mosquitoes are natural vectors of avian malaria in Africa. Malar J 8:193. doi:10.1186/1475-2875-8-193
Njabo KY, Cornel AJ, Bonneaud C, Toffelmier E, Sehgal RN, Valkiūnas G, Russell AF, Smith TB (2011) Nonspecific patterns of vector, host and avian malaria parasites associations in a central African rainforest. Mol Ecol 20:1049–1061. doi:10.1111/j.1365-294X.2010.04904.x
Nordling D, Anderson M, Zohari S, Gustafsson L (1998) Reproductive effort reduces specific immune response and parasite resistance. Proc R Soc Lond B 265:1291–1298
Olias PM, Wegelin W, Zenker S, Freter A, Gruber D, Klopfleisch R (2011) Avian malaria deaths in parrots, Europe. Emerg Infect Dis 17:950–952. doi:10.3201/eid1705.101618
Palinauskas V, Kosarev V, Shapoval A, Bensch S, Valkiūnas G (2007) Comparison of mitochondrial cytochrome b gene lineages and morphospecies of two avian malaria parasites of the subgenera of Haemamoeba and Giovannolaia (Haemosporida: Plasmodiidae). Zootaxa 1626:39–50
Palinauskas V, Valkiūnas G, Bolshakov CV, Bensch S (2008) Plasmodium relictum (lineage P-SGS1): effects on experimentally infected passerine birds. Exp Parasitol 120:372–380. doi:10.1016/j.exppara.2008.09.001
Peirce MA (1981) Distribution and host-parasite check-list of the haematozoa of birds in Western Europe. J Nat Hist 15:419–458
Santiago-Alarcon D, Outlaw DC, Ricklefs RE, Parker PG (2010) Phylogenetic relationships of haemosporidian parasites in New World Columbiformes, with emphasis on the endemic Galapagos dove. Int J Parasitol 40:463–470. doi:10.1016/j.ijpara.2009.10.003
Santiago-Alarcon D, Palinauskas V, Schaefer HH (2012) Diptera vectors of avian Haemosporidian parasites: untangling parasite life cycles and their taxonomy. Biol Rev 87:928–964. doi:10.1111/j.1469-185X.2012.00234.x
Schneider D, Shahabuddin M (2000) Malaria parasite development in a Drosophila model. Science 288:2376–2379
Sehgal RNM, Hull AC, Anderson NL, Valkiūnas G, Markovets MJ, Kawamura S, Tell LA (2006) Evidence for cryptic speciation of Leucocytozoon spp. (Haemosporida, Leucocytozoidae) in diurnal raptors. J Parasitol 92:375–379
Sinden RE (1998) Gametogenesis and sexual development. In: Sherman IW (ed) Malaria: parasite biology, pathogenesis, and protection. American Society for Microbiology Press, Washington, DC, pp 25–48
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC, Boca Raton
Valkiūnas G (2011) Haemosporidian vector research: marriage of molecular and microscopical approaches is essential. Mol Ecol 20:3084–3086. doi:10.1111/j.1365-294x.2011.05187.x
Valkiūnas G, Liutkevičius G, Iezhova TA (2002) Complete development of three species of Haemoproteus (Haemosporida, Haemoproteidae) in the biting midge Culicoides impunctatus (Diptera, Ceratopogonidae). J Parasitol 88:864–868
Valkiūnas G, Iezhova TA, Križanauskienė A, Palinauskas V, Bensch S (2008) A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J Parasitol 94:1395–1401. doi:10.1645/GE-1570.1
Valkiūnas G, Santiago-Alarcon D, Levin II, Iezhova TA, Parker PG (2010) A new Haemoproteus species (Haemosporida: Haemoproteidae) from the endemic Galapagos dove Zenaida galapagoensis, with remarks on the parasite distribution, vectors, and molecular diagnostics. J Parasitol 96:783–792. doi:10.1645/GE-2442.1
Valkiūnas G, Iezhova TA, Evans E, Carlson JS, Martínez-Gómez JE, Sehgal RNM (2013a) Two new Haemoproteus species (Haemosporida: Haemoproteidae) from columbiform birds. J Parasitol 99. doi:10.1645/12-98.1
Valkiūnas G, Palinauskas V, Križanauskienė A, Bernotienė R, Kazlauskienė R, Iezhova TA (2013b) Further observations on in vitro hybridization of hemosporidian parasites: patterns of ookinete development in Haemoproteus spp. J Parasitol 99:124–136. doi:10.1645/GE-3226.99
Ventim R, Ramos JA, Osório H, Lopes RJ, Pérez-Tris J, Mendes L (2012) Avian malaria infections in western European mosquitoes. Parasitol Res 111:637–645. doi:10.1007/S00436-012-2880-3
Acknowledgments
We would like to thank the staff of the Biological Station “Rybachy” for assistance in the field. The director of the Biological Station “Rybachy”, Casimir V. Bolshakov, is acknowledged for generously providing facilities for the experimental research. The experiments described herein comply with the current laws of Lithuania and Russia. The authors acknowledge the support of the Global Grant (VPI-3.1.-ŠMM-07-K-01-047).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valkiūnas, G., Kazlauskienė, R., Bernotienė, R. et al. Abortive long-lasting sporogony of two Haemoproteus species (Haemosporida, Haemoproteidae) in the mosquito Ochlerotatus cantans, with perspectives on haemosporidian vector research. Parasitol Res 112, 2159–2169 (2013). https://doi.org/10.1007/s00436-013-3375-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3375-6