Abstract
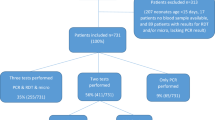
Though demonstration of Plasmodium parasite in peripheral blood on microscopy remains gold standard, it may miss some patients resulting in delay in instituting life-saving therapy. Studies on polymerase chain reaction (PCR), a highly sensitive and specific technique that also discriminates among different species of malaria parasite, are scanty. Hence, we aimed to evaluate the role of PCR in diagnosis and species identification of Plasmodium. Of 2186 febrile patients with clinical suspicion of malaria screened between July 2013 to February 2015, 561 patients fulfilled inclusion criteria. Microscopy, rapid diagnostic test (RDT) and PCR were performed to identify the parasite. Plasmodium was detected in 64/561 (11.40 %), 92/561 (16.40 %) and 78/561 (13.90 %) cases using microscopy, RDT and PCR, respectively. Of 78 positive cases by PCR, 47 (60.25 %) were confirmed as Plasmodium falciparum (P. falciparum), 28 (35.89 %) were Plasmodium vivax (P. vivax) and 3 (3.84 %) had mixed infections. Sensitivity and specificity of microscopy and RDT were 82.10 %, 100 % and 98.70 %, 96.90 %, respectively (p = 0.139). Of total 93 patients, 67 (72.04 %) were classified as complicated and 26 (27.96 %) were as uncomplicated. Creatinine (p = <0.001), conjugated bilirubin (p = 0.003) and total bilirubin (p = <0.001) level was elevated in complicated malaria along with renal (65 %) and liver dysfunction (25 %). In the present study, P. falciparum was responsible for 40/67 (59.70 %) cases of complicated malaria; P. vivax was also found in 17/67 (25.37 %) complicated cases using PCR. The findings highlight the alarming number of complicated vivax malaria in addition to falciparum. Moreover, PCR proved to be highly sensitive and specific test for detecting Plasmodium species.


Similar content being viewed by others
References
Abdallah TM, Abdeen MT, Ahmed IS, Hamdan HZ, Magzoub M, Adam I (2013) Severe Plasmodium falciparum and Plasmodium vivax malaria among adults at Kassala Hospital, eastern Sudan. Malar J 12:148. doi:10.1186/1475-2875-12-148
Andrade BB et al (2010) Towards a precise test for malaria diagnosis in the Brazilian Amazon: comparison among field microscopy, a rapid diagnostic test, nested PCR, and a computational expert system based on artificial neural networks. Malar J 9:117. doi:10.1186/1475-2875-9-117
Arevalo-Herrera M, Lopez-Perez M, Medina L, Moreno A, Gutierrez JB, Herrera S (2015) Clinical profile of Plasmodium falciparum and Plasmodium vivax infections in low and unstable malaria transmission settings of Colombia. Malar J 14:154. doi:10.1186/s12936-015-0678-3
Asma UE, Taufiq F, Khan W (2014) Prevalence and clinical manifestations of malaria in Aligarh, India. Korean J Parasitol 52:621–629. doi:10.3347/kjp.2014.52.6.621
Barker RH Jr, Banchongaksorn T, Courval JM, Suwonkerd W, Rimwungtragoon K, Wirth DF (1992) A simple method to detect Plasmodium falciparum directly from blood samples using the polymerase chain reaction. Am J Trop Med Hyg 46:416–426
Bartoloni A, Zammarchi L (2012) Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Dis 4:e2012026. doi:10.4084/MJHID.2012.026
Batwala V, Magnussen P, Nuwaha F (2010) Are rapid diagnostic tests more accurate in diagnosis of Plasmodium falciparum malaria compared to microscopy at rural health centres? Malar J 9:349. doi:10.1186/1475-2875-9-349
Bharti PK, Chand SK, Singh MP, Mishra S, Shukla MM, Singh R, Singh N (2013) Emergence of a new focus of Plasmodium malariae in forest villages of district Balaghat, Central India: implications for the diagnosis of malaria and its control. Trop Med Int Health 18:12–17. doi:10.1111/tmi.12005
Breman JG, Egan A, Keusch GT (2001) The intolerable burden of malaria: a new look at the numbers. Am J Trop Med Hyg 64:iv–vii
Brown AE, Kain KC, Pipithkul J, Webster HK (1992) Demonstration by the polymerase chain reaction of mixed Plasmodium falciparum and P. vivax infections undetected by conventional microscopy. Trans R Soc Trop Med Hyg 86:609–612
Fancony C, Sebastiao YV, Pires JE, Gamboa D, Nery SV (2013) Performance of microscopy and RDTs in the context of a malaria prevalence survey in Angola: a comparison using PCR as the gold standard. Malar J 12:284. doi:10.1186/1475-2875-12-284
Gougoutsi A, Karageorgopoulos DE, Dimitriadou A, Melas N, Kranidiotis G, Voutsinas D, Melidonis A (2014) Severe Plasmodium vivax malaria complicated with acute respiratory distress syndrome: a case associated with focal autochthonous transmission in Greece. Vector Borne Zoonotic Dis 14:378–381. doi:10.1089/vbz.2012.1192
Imwong M et al (2015) Plasma concentration of parasite DNA as a measure of disease severity in falciparum malaria. J Infect Dis 211:1128–1133. doi:10.1093/infdis/jiu590
Jain V et al (2008) Burden of cerebral malaria in central India (2004-2007). Am J Trop Med Hyg 79:636–642
Kato H, Nakagawa K, Tsuji T, Hamada T (1990) Subungual exostoses--clinicopathological and ultrastructural studies of three cases. Clin Exp Dermatol 15:429–432
Kochar D, Kumawat BL, Karan S, Kochar SK, Agarwal RP (1997) Severe and complicated malaria in Bikaner (Rajasthan), western India. Southeast Asian J Trop Med Public Health 28:259–267
Kochar DK et al (2009) Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg 80:194–198
Krishna S et al (2015) Detection of Mixed Infections with Plasmodium spp. by PCR, India, 2014. Emerg Infect Dis 21:1853–1857. doi:10.3201/eid2110.150678
Mackintosh CL, Beeson JG, Marsh K (2004) Clinical features and pathogenesis of severe malaria. Trends Parasitol 20:597–603. doi:10.1016/j.pt.2004.09.006
Mekonnen SK, Aseffa A, Medhin G, Berhe N, Velavan TP (2014) Re-evaluation of microscopy confirmed Plasmodium falciparum and Plasmodium vivax malaria by nested PCR detection in southern Ethiopia. Malar J 13:48. doi:10.1186/1475-2875-13-48
Mitra S, Abhilash K, Arora S, Miraclin A (2015) A prospective study from south India to compare the severity of malaria caused by Plasmodium vivax, P. falciparum and dual infection. J Vector Borne Dis 52:281–286
Mohanty S, Mishra SK, Pati SS, Pattnaik J, Das BS (2003) Complications and mortality patterns due to Plasmodium falciparum malaria in hospitalized adults and children, Rourkela, Orissa, India. Trans R Soc Trop Med Hyg 97:69–70
Mohapatra PK, Prakash A, Bhattacharyya DR, Goswami BK, Ahmed A, Sarmah B, Mahanta J (2008) Detection & molecular confirmation of a focus of Plasmodium malariae in Arunachal Pradesh, India. Indian J Med Res 128:52–56
Mwingira F, Genton B, Kabanywanyi AN, Felger I (2014) Comparison of detection methods to estimate asexual Plasmodium falciparum parasite prevalence and gametocyte carriage in a community survey in Tanzania. Malar J 13:433. doi:10.1186/1475-2875-13-433
Naik BS (2014) Incidence of jaundice in Plasmodium vivax malaria: a prospective study in Moodabidri, South India. Malays J Med Sci 21:24–27
Osman MM, Nour BY, Sedig MF, De Bes L, Babikir AM, Mohamedani AA, Mens PF (2010) Informed decision-making before changing to RDT: a comparison of microscopy, rapid diagnostic test and molecular techniques for the diagnosis and identification of malaria parasites in Kassala, eastern Sudan. Trop Med Int Health 15:1442–1448. doi:10.1111/j.1365-3156.2010.02659.x
Park TS, Oh SH, Choi JC, Kim HH, Chang CL, Son HC, Lee EY (2003) Plasmodium vivax malaria complicated by hemophagocytic syndrome in an immunocompetent serviceman. Am J Hematol 74:127–130. doi:10.1002/ajh.10390
Pinzon MA, Pineda JC, Rosso F, Shinchi M, Bonilla-Abadia F (2013) Plasmodium vivax cerebral malaria complicated with venous sinus thrombosis in Colombia. Asian Pac J Trop Med 6:413–415. doi:10.1016/S1995-7645(13)60050-4
Prakash J, Singh AK, Kumar NS, Saxena RK (2003) Acute renal failure in Plasmodium vivax malaria. J Assoc Physicians India 51:265–267
Rodulfo H, De Donato M, Mora R, Gonzalez L, Contreras CE (2007) Comparison of the diagnosis of malaria by microscopy, immunochromatography and PCR in endemic areas of Venezuela. Braz J Med Biol Res 40:535–543
Sambrook J, Russell DW (2006) Purification of nucleic acids by extraction with phenol:chloroform. CSH Protoc. doi:10.1101/pdb.prot4455
Sarkar D, Ray S, Saha M, Chakraborty A, Talukdar A (2013) Clinico-laboratory profile of severe Plasmodium vivax malaria in a tertiary care centre in Kolkata. Trop Parasitol 3:53–57. doi:10.4103/2229-5070.113912TP-3-53
Servonnet A, Rapp C, Delacour H, Bigaillon C, Pilo JE, Merens A (2012) Plasmodium knowlesi: an emerging species in humans? Med Sante Trop 22:417–421. doi:10.1684/mst.2012.0116
Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA (1999) A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg 60:687–692
Snounou G et al (1993) High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 61:315
Sonkar SK, Uniyal R, Sonkar GK (2011) Three unusual presentations of Plasmodium vivax malaria. Trop Doct 41:240–241. doi:10.1258/td.2011.110220
Tangpukdee N, Duangdee C, Wilairatana P, Krudsood S (2009) Malaria diagnosis: a brief review Korean. J Parasitol 47:93–102. doi:10.3347/kjp.2009.47.2.93
Warhurst DC, Williams JE (1996) ACP Broadsheet no 148. July 1996. Laboratory diagnosis of malaria. J Clin Pathol 49:533–538
WHO (2014) World Malaria Report 2014 WHO. http://www.appswhoint/iris/bitstream/10665/144852/2/9789241564830_engpdf. Accessed December 2014.
Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH (2007) A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg 77:119–127
Zakeri S et al (2010) Detection of mixed Plasmodium falciparum & P. vivax infections by nested-PCR in Pakistan, Iran & Afghanistan. Indian J Med Res 132:31–35
Acknowledgments
We would like to thank all the innocent patients for agreeing in the study and for donating their clinical samples. Staff members of our lab are also appreciated for helping in sample preparations and microscopic analyses. Mr. Prabhat Ranjan is grateful to Indian Council of Medical Research, New Delhi for providing the fellowship in conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
Here, we declare that we have no conflict of interests.
Rights and permissions
About this article
Cite this article
Ranjan, P., Ghoshal, U. Utility of nested polymerase chain reaction over the microscopy and immuno-chromatographic test in the detection of Plasmodium species and their clinical spectrum. Parasitol Res 115, 3375–3385 (2016). https://doi.org/10.1007/s00436-016-5098-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5098-y