Abstract
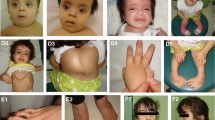
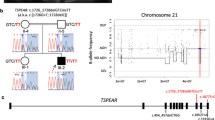
Biallelic mutations of UBE3B have recently been shown to cause Kaufman oculocerebrofacial syndrome (also reported as blepharophimosis–ptosis–intellectual disability syndrome), an autosomal recessive condition characterized by hypotonia, developmental delay, intellectual disability, congenital anomalies, characteristic facial dysmorphic features, and low cholesterol levels. To date, six patients with either missense mutations affecting the UBE3B HECT domain or truncating mutations have been described. Here, we report on the identification of homozygous or compound heterozygous UBE3B mutations in six additional patients from five unrelated families using either targeted UBE3B sequencing in individuals with suggestive facial dysmorphic features, or exome sequencing. Our results expand the clinical and mutational spectrum of the UBE3B-related disorder in several ways. First, we have identified UBE3B mutations in individuals who previously received distinct clinical diagnoses: two sibs with Toriello–Carey syndrome as well as the patient reported to have a “new” syndrome by Buntinx and Majewski in 1990. Second, we describe the adult phenotype and clinical variability of the syndrome. Third, we report on the first instance of homozygous missense alterations outside the HECT domain of UBE3B, observed in a patient with mildly dysmorphic facial features. We conclude that UBE3B mutations cause a clinically recognizable and possibly underdiagnosed syndrome characterized by distinct craniofacial features, hypotonia, failure to thrive, eye abnormalities, other congenital malformations, low cholesterol levels, and severe intellectual disability. We review the UBE3B-associated phenotypes, including forms that can mimick Toriello–Carey syndrome, and suggest the single designation “Kaufman oculocerebrofacial syndrome”.

Similar content being viewed by others
References
Al Frayh AR, Haque KN (1987) Anophthalmia, microcephaly, hypotonia, hypogonadism, failure to thrive and developmental delay. Dysmorph Clin Genet 1:64–66
Basel-Vanagaite L, Dallapiccola B, Ramirez-Solis R, Segref A, Thiele H, Edwards A, Arends MJ, Miró X, White JK, Désir J, Abramowicz M, Dentici ML, Lepri F, Hofmann K, Har-Zahav A, Ryder E, Karp NA, Estabel J, Gerdin AK, Podrini C, Ingham NJ, Altmüller J, Nürnberg G, Frommolt P, Abdelhak S, Pasmanik-Chor M, Konen O, Kelley RI, Shohat M, Nürnberg P, Flint J, Steel KP, Hoppe T, Kubisch C, Adams DJ, Borck G (2012) Deficiency for the ubiquitin ligase UBE3B in a blepharophimosis–ptosis–intellectual-disability syndrome. Am J Hum Genet 91(6):998–1010. doi:10.1016/j.ajhg.2012.10.011
Buntinx I, Majewski F (1990) Blepharophimosis, iris coloboma, microgenia, hearing loss, postaxial polydactyly, aplasia of corpus callosum, hydroureter, and developmental delay. Am J Med Genet 36(3):273–274. doi:10.1002/ajmg.1320360304
Clayton-Smith J, O’Sullivan J, Daly S, Bhaskar S, Day R, Anderson B, Voss AK, Thomas T, Biesecker LG, Smith P, Fryer A, Chandler KE, Kerr B, Tassabehji M, Lynch SA, Krajewska-Walasek M, McKee S, Smith J, Sweeney E, Mansour S, Mohammed S, Donnai D, Black G (2011) Whole-exome-sequencing identifies mutations in histone acetyltransferase gene KAT6B in individuals with the Say-Barber-Biesecker variant of Ohdo syndrome. Am J Hum Genet 89(5):675–681. doi:10.1016/j.ajhg.2011.10.008
Dentici ML, Mingarelli R, Dallapiccola B (2011) The difficult nosology of blepharophimosis–mental retardation syndromes: report on two siblings. Am J Med Genet A 155A(3):459–465. doi:10.1002/ajmg.a.33642
Figuera LE, García-Cruz D, Ramírez-Dueñas ML, Rivera-Robles V, Cantù JM (1993) Kaufman oculocerebrofacial syndrome: report of two new cases and further delineation. Clin Genet 44(2):98–101. doi:10.1111/j.1399-0004.1993.tb03855.x
Flex E, Ciolfi A, Caputo V, Fodale V, Leoni C, Melis D, Bedeschi MF, Mazzanti L, Pizzuti A, Tartaglia M, Zampino G (2013) Loss of function of the E3 ubiquitin-protein ligase UBE3B causes Kaufman oculocerebrofacial syndrome. J Med Genet 50(8):493–499. doi:10.1136/jmedgenet-2012-101405
Gabrielli O, Ficcadenti A, Fabrizzi G, Perri P, Mercuri A, Coppa GV, Giorgi P (1994) Child with oral, facial, digital, and skeletal anomalies and psychomotor delay: a new OFDS form? Am J Med Genet 53(3):290–293. doi:10.1002/ajmg.1320530315
Gandomi SK, Farwell Gonzalez KD, Parra M, Shahmirzadi L, Mancuso J, Pichurin P, Temme R, Dugan S, Zeng W, Tang S (2013) Diagnostic exome sequencing identifies two novel IQSEC2 mutations associated with X-linked intellectual disability with seizures: implications for genetic counseling and clinical diagnosis. J Genet Couns
Garcia-Cruz D, Arreola R, Sanchez-Corona J, Garcia-Cruz O, Renteria R, Villar V, Gonzalez ME, Vargas-Moyeda E, Cantu JM (1988) Kaufman oculocerebrofacial syndrome: a corroborative report. Dysmorph Clin Genet 1:152–154
Gong TW, Huang L, Warner SJ, Lomax MI (2003) Characterization of the human UBE3B gene: structure, expression, evolution, and alternative splicing. Genomics 82(2):143–152. doi:10.1016/S0888-7543(03)00111-3
Hansen L, Tawamie H, Murakami Y, Mang Y, Rehman S, Buchert R, Schaffer S, Muhammad S, Bak M, Nöthen MM, Bennett EP, Maeda Y, Aigner M, Reis A, Kinoshita T, Tommerup N, Baig SM, Abou Jamra R (2013) Hypomorphic mutations in PGAP2, encoding a GPI-anchor-remodeling protein, cause autosomal-recessive intellectual disability. Am J Hum Genet 92(4):575–583. doi:10.1016/j.ajhg.2013.03.008
Hirst JD, Vieth M, Skolnick J, Brooks CL 3rd (1996) Predicting leucine zipper structures from sequence. Protein Eng 9(8):657–662. doi:10.1093/protein/9.8.657
Kaufman RL, Rimoin DL, Prensky AL, Sly WS (1971) An oculocerebrofacial syndrome. Birth Defects Orig Artic Ser 7(1):135–138
Kleefstra T, Brunner HG, Amiel J, Oudakker AR, Nillesen WM, Magee A, Geneviève D, Cormier-Daire V, van Esch H, Fryns JP, Hamel BC, Sistermans EA, de Vries BB, van Bokhoven H (2006) Loss-of-function mutations in euchromatin histone methyl transferase 1 (EHMT1) cause the 9q34 subtelomeric deletion syndrome. Am J Hum Genet 79(2):370–377
Ropers F, Derivery E, Hu H, Garshasbi M, Karbasiyan M, Herold M, Nürnberg G, Ullmann R, Gautreau A, Sperling K, Varon R, Rajab A (2011) Identification of a novel candidate gene for non-syndromic autosomal recessive intellectual disability: the WASH complex member SWIP. Hum Mol Genet 20(13):2585–2590. doi:10.1093/hmg/ddr158
Toriello HV, Carey JC (1988) Corpus callosum agenesis, facial anomalies, Robin sequence, and other anomalies: a new autosomal recessive syndrome? Am J Med Genet 31(1):17–23. doi:10.1002/ajmg.1320310105
Toriello HV, Carey JC, Addor MC, Allen W, Burke L, Chun N, Dobyns W, Elias E, Gallagher R, Hordijk R, Hoyme G, Irons M, Jewett T, LeMerrer M, Lubinsky M, Martin R, McDonald-McGinn D, Neumann L, Newman W, Pauli R, Seaver L, Tsai A, Wargowsky D, Williams M, Zackai E (2003) Toriello–Carey syndrome: delineation and review. Am J Med Genet A 123A(1):84–90. doi:10.1002/ajmg.a.20493
Verloes A, Bremond-Grignac D, Isidor B, David A, Baumann C, Leroy M-A, Stevens R, Gillerot Y, Héron D, Héron B, Benzacken B, Lacombe D, Brunner H, Bitoun P (2006) Blepharophimosis–mental retardation (BMR) syndromes: a proposed clinical classification of the so-called Ohdo syndrome, and delineation of two new BMR syndromes, one X-linked and one autosomal recessive. Am J Med Genet A 140(12):1285–1296. doi:10.1002/ajmg.a.31270
Vulto-van Silfhout AT, de Vries BB, van Bon BW, Hoischen A, Ruiterkamp-Versteeg M, Gilissen C, Gao F, van Zwam M, Harteveld CL, van Essen AJ, Hamel BC, Kleefstra T, Willemsen MA, Yntema HG, van Bokhoven H, Brunner HG, Boyer TG, de Brouwer AP (2013) Mutations in MED12 cause X-linked Ohdo syndrome. Am J Hum Genet 92(3):401–406. doi:10.1016/j.ajhg.2013.01.007
Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, Braxton A, Beuten J, Xia F, Niu Z, Hardison M, Person R, Bekheirnia MR, Leduc MS, Kirby A, Pham P, Scull J, Wang M, Ding Y, Plon SE, Lupski JR, Beaudet AL, Gibbs RA, Eng CM (2013) Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med 369(16):1502–1511. doi:10.1056/NEJMoa1306555
Acknowledgments
We wish to thank the family members for their participation, Gabrielle J. Halpern for help with editing the manuscript, and H. Toriello, M. C. Addor, R. Hordijk, N. Dikow and K. van Berkel for contributing DNA from patients with TCS. This work was supported by the Israeli Ministry of Health Chief Scientist foundation (grant No. 3-4963) and Israeli Science Foundation (grant No. 09/558) (L.B.-V.). G.B. was supported by the Deutsche Forschungsgemeinschaft. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
L. Basel-Vanagaite and R. Yilmaz contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
439_2014_1436_MOESM1_ESM.tif
Supplementary Figure 1 MRI scans of six individuals with biallelic UBE3B mutations. Patient 1 (MRI performed at the age of 32 years): moderate dilatation of the lateral ventricles; Patient 2 (14 months): slightly dilated lateral ventricles and thin corpus callosum; Patient 3 (newborn period): frontal lipoma, short corpus callosum; Patient 4 (7 years): no gross anomalies; Patient 5 (8 years): severe dilatation of the lateral ventricles, Chiari type 1 malformation; Patient 10 (10 days): severe hypoplasia (or agenesis) of the corpus callosum (TIFF 7663 kb)
Rights and permissions
About this article
Cite this article
Basel-Vanagaite, L., Yilmaz, R., Tang, S. et al. Expanding the clinical and mutational spectrum of Kaufman oculocerebrofacial syndrome with biallelic UBE3B mutations. Hum Genet 133, 939–949 (2014). https://doi.org/10.1007/s00439-014-1436-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-014-1436-2