Abstract
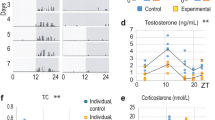
The Leydig cell is the primary source of testosterone in males. Levels of testosterone in circulation are determined by the steroidogenic capacities of individual Leydig cells and the total numbers of Leydig cells per testis. Stress-induced increases in serum glucocorticoid concentrations inhibit testosterone-biosynthetic enzyme activity, leading to decreased rates of testosterone secretion. It is unclear, however, whether the excessive glucocorticoid stimulation also affects total Leydig cell numbers through induction of apoptosis and thereby contributes to the stress-induced suppression of androgen levels. Exposure of Leydig cells to high concentrations of corticosterone (CORT, the endogenously secreted glucocorticoid in rodents) increases their frequency of apoptosis. Studies of immobilization stress indicate that stress-induced increases in CORT are directly responsible for Leydig cell apoptosis. Access to glucocorticoid receptors in Leydig cells is modulated by oxidative inactivation of glucocorticoid by 11β-hydroxysteroid dehydrogenase (11βHSD). Under basal levels of glucocorticoid, sufficient levels of glucocorticoid metabolism occur and there is likely to be minimal binding of the glucocorticoid receptor. We have established that Leydig cells express type 1 11βHSD, an oxidoreductase, and type 2, a unidirectional oxidase. Generation of redox potential through synthesis of the enzyme cofactor NADPH, a byproduct of glucocorticoid metabolism by 11βHSD-1, may potentiate testosterone biosynthesis, as NADPH is the cofactor used by steroidogenic enzymes such as type 3 17β-hydroxysteroid dehydrogenase. In this scenario, inhibition of steroidogenesis will only occur under stressful conditions when high input amounts of CORT exceed the capacity of oxidative inaction by 11βHSD. Changes in autonomic catecholaminergic activity may contribute to suppressed Leydig cell function during stress, and may explain the rapid onset of inhibition. However, recent analysis of glucocorticoid action in Leydig cells indicates the presence of a fast, non-genomic pathway that will merit further investigation.




Similar content being viewed by others
References
Agarwal AK, Mune T, Monder C, White PC (1994) NAD(+)-dependent isoform of 11β-hydroxysteroid dehydrogenase. Cloning and characterization of cDNA from sheep kidney. J Biol Chem 269:25959–25962
Almeida SA, Anselmo-Franci JA, Silva AAR, Carvalho TL (1998) Chronic intermittent immobilization of male rats throughout sexual development: a stress protocol. Exp Physiol 83:701–704
Baldwin DM (1979) Effects of glucocorticoids on estrogen-dependent LH release in the ovariectomized rat and on gonadotropin secretion in the intact female rat. Endocrinology 105:120–178
Barbazanges A, Piazza PV, LeMoal M, Maccari S (1996) Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci 16:3943–3949
Bernier M, Gibb W, Collu R, Ducharme JR (1984) Effect of glucocorticoids on testosterone production by porcine Leydig cells in primary culture. Can J Physiol Pharmacol 62:1166–1169
Breuiller M, Tahri-Joutei A, Ferre F, Pointis G (1988) Beta-adrenergic receptors and stimulatory effects of (-) isoproterenol on testosterone production in fetal mouse Leydig cells. Biochem Biophys Res Commun 151:1454–1460
Chrousos GP, Gold PW (1992) The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267:1244–1252
Contreras LN, Masini AM, Danna MM, Kral M, Bruno OD, Rossi MA, Andrada JA (1996) Glucocorticoids: their role on gonadal function and LH secretion. Minerva Endocrinol 21:43–46
Dong Q, Salva A, Sottas CM, Niu E, Holmes M, Hardy MP (2004) Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J Androl 25:972–981
Fenster L, Katz DF, Wyrobek AJ, Pieper C, Rempel DM, Oman D, Swan SH (1997) Effects of psychological stress on human semen quality. J Androl 18:194–202
Ferguson SE, Pallikaros Z, Michael AE, Cooke BA (1999) The effects of different culture media, glucose, pyridine nucleotides and adenosine on the activity of 11β-hydroxysteroid dehydrogenase in rat Leydig cells. Mol Cell Endocrinol 158:37–44
Gao HB, Tong MH, Hu YQ, You HY, Guo QS, Ge RS, Hardy MP (2003) Mechanisms of glucocorticoid-induced Leydig cell apoptosis. Mol Cell Endocrinol 199:153–163
Gasser PJ, Orchinik M (2000) Membrane receptors for glucocorticoids. In: Fink G (ed) Encyclopedia of stress. Academic, New York pp 713–721
Ge RS, Hardy MP (2002) Protein kinase C increases 11β-hydroxysteroid dehydrogenase oxidation and inhibits reduction in rat Leydig cells. J Androl 23:135–143
Ge RS, Gao HB, Nacharaju VL, Gunsalus GL, Hardy MP (1997) Identification of a kinetically distinct activity of 11β-hydroxysteroid dehydrogenase in rat Leydig cells. Endocrinology 138:2435–2442
Ge R-S, Dong Q, Niu E-M, Sottas CM, Hardy DO, Catterall JF, Latif SA, Morris DJ, Hardy MP (2005) 11β-Hydroxysteroid dehydrogenase 2 in rat leydig cells: its role in blunting glucocorticoid action at physiological levels of substrate. Endocrinology en.2005-0046
Hales DB, Payne AH (1989) Glucocorticoid-mediated repression of P450scc mRNA and de novo synthesis in cultured Leydig cells. Endocrinology 124:2099–21104
Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, McEwen BS, Haider SG, Markham CM, Blanchard RJ, Blanchard DC, Sakai RR (2002) Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol Reprod 67:1750–1755
Hyde GN, Seale AP, Grau EG, Borski RJ (2004) Cortisol rapidly suppresses intracellular calcium and voltage-gated calcium channel activity in prolactin cells of the tilapia (Oreochromis mossambicus). Am J Physiol Endocrinol Metab 286:E626–E633
Kim JM, Luo L, Zirkin BR (2000) Caspase-3 activation is required for Leydig cell apoptosis induced by ethane dimethanesulfonate. Endocrinology 141:1846–1853
King KL, Cidlowski JA (1998) Cell cycle regulation and apoptosis. Annu Rev Physiol 60:601–617
Lakshmi V, Monder C (1988) Purification and characterization of the corticosteroid 11β-dehydrogenase component of the rat liver 11β-hydroxysteroid dehydrogenase complex. Endocrinology 123:2390–2398
Leckie CM, Welberg LA, Seckl JR (1998) 11β-Hydroxysteroid dehydrogenase is a predominant reductase in intact rat Leydig cells. J Endocrinol 159:233–238
Lee S, Miselis R, Rivier C (2002) Anatomical and functional evidence for a neural hypothalamic-testicular pathway that is independent of the pituitary. Endocrinology 143:4447–4454
Martinsons A, Rudzite V, Bratslavska O, Saulite V (1999) The influence of kynurenine, neopterin, and norepinephrine on tubular epithelial cells and alveolar fibroblasts. Adv Exp Med Biol 467:347–352
McEwen BS, Albeck D, Cameron H, Chao HM, Gould E, Hastings N, Kuroda Y, Luine V, Magarinos AM, McKittrick CR (1995) Stress and the brain: a paradoxical role for adrenal steroids. Vitam Horm 51:371–402
Monder C, Shackleton CH (1984) 11β-Hydroxysteroid dehydrogenase: fact or fancy? Steroids 44:383–417
Monder C, Sakai RR, Miroff Y, Blanchard DC, Blanchard RJ (1994a) Reciprocal changes in plasma corticosterone and testosterone in stressed male rats maintained in a visible burrow system: evidence for a mediating role of testicular β-hydroxysteroid dehydrogenase. Endocrinology 134:1193–1198
Monder C, Hardy MP, Blanchard RJ, Blanchard DC (1994b) Comparative aspects of 11β-hydroxysteroid dehydrogenase. Testicular 11β-hydroxysteroid dehydrogenase: development of a model for the mediation of Leydig cell function by corticosteroids. Steroids 59:69–73
Morris ID, Lendon RG, Waters C, Naylor G, Jones N (1997) Thymic regression and apoptosis in the rat after treatment with the Leydig cell cytotoxin ethylene dimethanesulphonate (EDS). Toxicology 120:19–27
Moutsatsou P, Psarra A-MG, Tsiapara A, Paraskevakou H, Davaris P, Sekeris CE (2001) Localization of the glucocorticoid receptor in rat brain mitochondria. Arch Biochem Biophys 386:69–78
Munck A, Guyre PM, Holbrook NJ (1984) Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrinol Rev 5:25–43
Nakashima A, Koshiyama K, Uozumi T, Monden Y, Hamanaka Y, Kurachi K, Aono T, Mizutani S, Matsumoto K (1975) Effects of general anaesthesia and severity of surgical stress on serum LH and testosterone in males. Acta Endocrinol 78:258–269
Norman RL (1993) Effects of corticotropin-releasing hormone on luteinizing hormone, testosterone, and cortisol secretion in intact male rhesus macaques. Biol Reprod 49:148–153
Orr TE, Taylor MF, Bhattacharyya AK, Collins DC, Mann DR (1994) Acute immobilization stress disrupts testicular steroidogenesis in adult male rats by inhibiting the activities of 17α-hydroxylase and 17,20-lyase without affecting the binding of LH/hCG receptors. J Androl 15:302–308
Payne AH, Sha LL (1991) Multiple mechanisms for regulation of 3β-hydroxysteroid dehydrogenase/D5-D4-isomerase, 17α-hydroxylase/C17-20 lyase cytochrome P450, and cholesterol side-chain cleavage cytochrome P450 messenger ribonucleic acid levels in primary cultures of mouse Leydig cells. Endocrinology 129:1429–1435
Phillips DM, Lakshmi V, Monder C (1989) Corticosteroid 11β-dehydrogenase in rat testi. Endocrinology 125:209–216
Romeo F, Li D, Shi M, Mehta JL (2000) Carvedilol prevents epinephrine-induced apoptosis in human coronary artery endothelial cell: modulation of Fas/FasL ligand and caspase-3 pathway. Cardiovasc Res 45:788–794
Rusvai E, Naray-Fejes-Toth A (1993) A new isoform of 11β-hydroxysteroid dehydrogenase in aldosterone target cells. J Biol Chem 268:10717–10720
Sapolsky RM (ed) (1992) Stress, the aging brain, and the mechanisms of neuron death. MIT Press, Cambridge, Mass., 429 pp
Schwartzman RA, Cidlowski JA (1993) Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocrine Rev 14:133–151
Selye H (1946) The general adaptation syndrome and the disease of adaptation. J Clin Endocrinol 6:117–230
Stalker A, Hermo L, Antakly T (1989) Covalent affinity labeling, radioautography, and immunocytochemistry localize the glucocorticoid receptor in rat testicular Leydig cells. Am J Anat 186:369–377
Stalker A, Hermo L, Antakly T (1991) Subcellular distribution of 3H-dexamethasone mesylate binding sites in Leydig cells using electron microscope radioautography. Am J Anat 190:19–30
Suter DE, Schwartz NB (1985) Effects of glucocorticoids on secretion of luteinizing hormone and follicle-stimulating hormone by female rat pituitary cell in vitro. Endocrinology 117:849–854
Tohei A, Tomabechi T, Mamada M, Akai M, Watanabe G, Taya K (1997) Effects of repeated ether stress on the hypothalamic-pituitary-testes axis in adult rats with special reference to inhibin secretion. J Vet Med Sci 59:329–334
Vierhapper H, Nowotny P, Waldhausl W (2000) Production rates of testosterone in patients with Cushing's syndrome. Metab Clin Exp 49:229–231
White PC, Mune T, Agarwal AK (1997) 11β-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocrine Rev 18:135–156
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hardy, M.P., Gao, HB., Dong, Q. et al. Stress hormone and male reproductive function. Cell Tissue Res 322, 147–153 (2005). https://doi.org/10.1007/s00441-005-0006-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-005-0006-2