Abstract
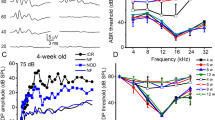
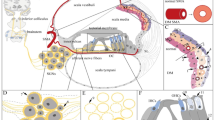
Hearing loss secondary to diabetes remains under debate. In our study, we used Zucker Diabetic Fatty (ZDF) rats as an animal model of type 2 diabetes to investigate whether (1) hearing ability impairment and structural alterations of the inner ear occur in diabetes and (2) an angiotensin II receptor blocker (losartan) can protect rats from diabetic damage. Homozygous mutants were treated with a placebo or losartan and heterozygous animals served as non-diabetic controls. All animals underwent immunohistochemical and electronmicroscopical analysis. Functional testing of hearing ability was performed by click-evoked auditory brainstem responses. The present study showed significant sensorineural hearing impairment in placebo-treated diabetic rats (hearing threshold, 45.0 ± 2.1 dB SPL) compared to both non-diabetic controls (34.7 ± 4 dB SPL) and losartan-treated diabetic rats (36.1 ± 7.4 dB SPL). Concurrently, the functional decline in the placebo-treated rats was associated with significant morphological abnormalities, particularly in the intermediate cells of the stria vascularis and with strial dysfunction. These degenerative changes were indicated by the down-regulation of several pumps, ionic and cellular channels, which are involved in the cycling of K+ and the maintenance of the endocochlear potential essential for the hearing process. Thus, the inner ear can be regarded as a target organ during hyperglycemic disorders and a metabolically induced “diabetic otopathy” may be added to angiopathy, nephropathy and neuropathy as a specific complication of diabetes mellitus. Blockade of the angiotensin II receptor can prevent this “diabetic otopathy” despite hyperglycemic serum levels.




Similar content being viewed by others
Abbreviations
- ABR:
-
Auditory brainstem responses
- BC:
-
Basal cell
- Cx:
-
Connexin
- dB:
-
Decibel
- EP:
-
Endocochlear potential
- IMC:
-
Intermediate cell
- MC:
-
Marginal cell
- RAS:
-
Renin-angiotensin system
- SV:
-
Stria vascularis
- T2DM:
-
Type 2 diabetes mellitus
- ZDF:
-
Zucker Diabetic Fatty Rats
References
Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD (2011) Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A 108:14849–14854
Akinpelu OV, Mujica-Mota M, Daniel SJ (2013) Is type 2 diabetes mellitus associated with alterations in hearing?: a systematic review and meta-analysis. Laryngoscope 133:767–776
Aladag I, Eyibilen A, Güven M, Atis O, Erkorkmaz Ü (2009) Role of oxidative stress in hearing impairment in patients with type two diabetes mellitus. J Laryngol Otol 123:957–963
Cao Z, Cooper ME (2011) Efficacy of renin-angiotensin system (RAS) blockers on cardiovascular and renal outcomes in patients with type 2 diabetes. Acta Diabetol 49:243–254
Cone RD (2005) Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578
De Cavanagh EMV, Piotrkowski B, Basso N, Stella I, Inserra F, Ferder L, Fraga CD (2003) Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. FASEB J 17:1096–1098
Duman D, Tekin M (2012) Autosomal recessive nonsyndromic deafness genes: a review. Front Biosci 17:2213–2236
Eckhard A, Gleiser C, Rask-Andersen H, Arnold H, Liu W, Mack A, Müller M, Löwenheim H, Hirt B (2012) Co-localisation of Kir4.1 and AQP4 in rat and human cochleae reveals a gap in water channel expression at the transduction sites of endocochlear K+ recycling routes. Cell Tissue Res 350:27–43
Forge A, Becker D, Casalotti S, Edwards J, Marziano N, Nevill G (2003) Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J Comp Neurol 467:207–231
Frisina ST, Mapes F, Kim S, Frisina DR, Frisina RD (2006) Characterization of hearing loss in aged type II diabetics. Hear Res 211:103–113
Fukushima H, Cureoglu S, Schachern PA, Paparella MM, Harada T, Oktay MF (2006) Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch Otolaryngol Head Neck Surg 132:934–938
Gratton M, Smyth BJ, Schulte BA, Vincent DA Jr (1995) Na, K-ATPase activity decreases in the cochlear lateral wall of quiet-aged gerbils. Hear Res 83:43–50
Hamelin R, Allagnat F, Haefliger JA, Meda P (2009) Connexins, diabetes and the metabolic syndrome. Curr Protein Pept Sci 10:18–29
Hibino H, Kurach Y (2006) Molecular and physiological bases of the K+ circulation in the mammalian inner ear. Physiology 21:336–345
Huh SK, Lipton JM, Batjer HH (1997) The protective effects of alpha-melanocyte stimulating hormone on canine brain stem ischemia. Neurosurgery 40:132–140
Jordao AMD (1857) Considérations sur un cas de diabéte. These, Faculte de Medicine de Paris
Leem J, Koh EH (2012) Interaction between mitochondria and the endoplasmic reticulum: Implications for the pathogenesis of Type 2 diabetes mellitus. Exp Diabetes Res 2012:242984
Maia CA, Campos CA (2005) Diabetes mellitus as etiological factor of hearing loss. Braz J Otorhinolaryngol 71:208–214
Marcus DC, Wu T, Wangemann P, Kofuji P (2002) KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol Cell Physiol 282:C403–C407
McQueen CT, Baxter A, Smith TL, Raynor E, Yoon SM, Prazma J, Pillsbury HC 3rd (1999) Non-insulin-dependent diabetic microangiopathy in the inner ear. J Laryngol Otol 113:13–18
Meyer zum Gottesberge AM (1988) Physiology and pathophysiology of inner ear melanin. Pigment Cell Res 1:238–249
Meyer zum Gottesberge AM (2000) Hormonal regulation of the inner ear. In: Sterkers O, Ferrary E, Dauman R, Sauvage JP, Tran Ba Huy P (eds) Menière’s disease 1999–Update. Kugler, The Hague, pp 49–57
Meyer zum Gottesberge AM (2005) Alpha-MSH as an emergency hormone of the inner ear? ARO 1134 (Abstract)
Meyer zum Gottesberge AM, Massing T, Hansen S (2012) Missing mitochondrial Mpv17 gene function induces tissue-specific cell-death pathway in the degenerating inner ear. Cell Tissue Res 347:343–356
Murillo-Cuesta S, Camarero G, González-Rodríguez A, De La Rosa LR, Burks DJ, Avendaño C, Valverde AM, Varela-Nieto I (2012) Insulin receptor substrate 2 (IRS2)-deficient mice show sensorineural hearing loss that is delayed by concomitant protein tyrosine phosphatase 1B (PTP1B) loss of function. Mol Med 18:260–269
Nakae S, Tachibana M (1986) The cochlea of the spontaneously diabetic mouse. II. Electron microscopic observations of non-obese diabetic mice. Arch Otorhinolaryngol 243:313–316
Ohlemiller KK, Rice MER, Gagnon PM (2008) Strial microvascular pathology and age-associated endocochlear potential decline in NOD congenic mice. Hear Res 244:85–97
Patuzzi R (2011) Ion flow in cochlear hair cells and the regulation of hearing sensitivity. Hear Res 280:3–20
Raynor E, Robison WG, Garrett CG, McGuirt WT, Pillsbury HC, Prazma J (1995) Consumption of a high-galactose diet induces diabetic-like changes in the inner ear. Otolaryngol Head Neck Surg 113:748–754
Ribeiro-Oliveira A Jr, Nogueira AI, Pereira RM, Boas WW, Dos Santos RA, Simões e Silva AC (2008) The renin–angiotensin system and diabetes: an update. Vasc Health Risk Manag 4:787–803
Rozengurt N, Lopez I, Chiu CS, Kofuji P, Lester HA, Neusch C (2003) Time course of inner ear degeneration and deafness in mice lacking the Kir4.1 potassium channel subunit. Hear Res 177:71–80
Rust KR, Prazma J, Triana RJ, Michaelis OE 4th, Pillsbury HC (1992) Inner Ear damage secondary to diabetes mellitus: II. Changes in aging SHR/N-cp rats. Arch Otolaryngol Head Neck Surg 118:397–400
Ryan AF, Wattsm AG (1991) Expression of mRNAs encoding α and β subunit isoforms of the Na, K-ATPase in the rat cochlea. Mol Cell Neurosci 2:179–187
Shafrir E (2010) Contribution of animal models to the research of the causes of diabetes. World J Diabetes 1:137–140
Silva KC, Rosales MAB, Biswas SK, Lopes de Faria JB, Lopes de Faria JM (2009) Diabetic retinal neurodegeneration is associated with mitochondrial oxidative stress and is improved by an angiotensin receptor blocker in a model combining hypertension and diabetes. Diabetes 58:1382–1390
Smith TL, Raynor E, Prazma J, Buenting JE, Pillsbury HC (1995) Insulin-dependent diabetic microangiopathy in the inner ear. Laryngoscope 105:236–240
Spicer SS, Schulte BA (2005) Novel structures in marginal and intermediate cells presumably relate to functions of apical versus basal strial strata. Hear Res 200:87–101
Spicer SS, Gratton MA, Schulte BA (1997) Expression patterns of ion transport enzymes in spiral ligament fibrocytes change in relation to strial atrophy in the aged gerbil cochlea. Hear Res 111:93–102
Srinivasan K, Ramarao P (2007) Animal models in type 2 diabetes research: an overview. Indian J Med Res 125:451–471
Tachibana M, Nakae S (1986) The cochlea of the spontaneously diabetic mouse. I. Electron microscopic observation of KK mice. Arch Otorhinolaryngol 243:238–241
Tocci G, Volpe M (2011) End-organ protection in patients with hypertension. Drugs 71:1003–1017
Tomisawa H (2000) Diabetic changes in the stria vascularis in humans–a study of PAS-stained temporal bone sections. Nihon Jibiinkoka Gakkai Kaiho 103:1227–1237
Tóth F, Várkonyi TT, Kiss JG, Rovó L, Lengyel C, Légrády P, Jór J, Czigner J (2001) Brainstem auditory-evoked potential examinations in diabetic patients. Scand Audiol Suppl 52:156–159
Triana RJ, Suits GW, Garrison S, Prazma J, Brechtelsbauer PB, Michaelis OE, Pillsbury HC (1991) Inner Ear damage secondary to diabetes mellitus: I. Changes in adolescent SHR/N-cp rats. Arch Otolaryngol Head Neck Surg 117:635–640
Vague P, Coste TC, Jannot MF, Raccah D, Tsimaratos M (2004) C-peptide, Na+, K + -ATPase, and Diabetes. Exper Diabesity Res 5:37–50
Wolters FL, Klis SF, Hamers FP, de Groot JC, Smoorenburg GF (2004) Perilymphatic application of alpha-melanocyte stimulating hormone ameliorates hearing loss caused by systemic administration of cisplatin. Hear Res 189:31–40
Wright JA, Richards T, Becker DL (2012) Connexins and diabetes. Cardiol Res Pract 2012:496904
Xie B, Jiao Q, Cheng Y, Zhong Y, Shen X (2012) Effect of pigment epithelium-derived factor on glutamate uptake in retinal muller cells under high-glucose conditions. Invest Ophthalmol Vis Sci 53:1023–1032
Acknowledgments
We wish to thank Prof. S. Schäfer (Sanofi/Aventis) for initiating the study and providing the material and Mr. P. Hainz and G. Fischer (Sanofi/Aventis) and Mrs. U. Becker-Ledzian (Düsseldorf) for outstanding technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meyer zum Gottesberge, AM., Massing, T., Sasse, A. et al. Zucker diabetic fatty rats, a model for type 2 diabetes, develop an inner ear dysfunction that can be attenuated by losartan treatment. Cell Tissue Res 362, 307–315 (2015). https://doi.org/10.1007/s00441-015-2215-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2215-7