Abstract
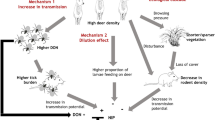
Deer mice (Peromyscus maniculatus) are the primary reservoir for Sin Nombre virus (SNV), a North American hantavirus that causes disease with high mortality in humans. Recent studies have proposed that habitat disturbance affects prevalence of SNV in deer mice; however, the outcomes proposed in these studies are in opposition to each other. Our objectives were to test these divergent hypotheses by: (1) measuring SNV infection in deer mice within a patchwork of disturbance; and (2) evaluating the relationships between SNV prevalence, population density and demography as possible mechanisms. In 2003 and 2004, we sampled 1,297 deer mice from 17 sites with varying levels of disturbance in the Great Basin Desert. Across sites and years, SNV prevalence varied from 0.0 to 38.9%. We found a negative relationship between SNV prevalence and disturbance. Although we found no direct relationship between SNV prevalence and deer mouse density, we found that density was highest on sites with the lowest levels of disturbance. The number of deer mice that survived across seasons (e.g., trans-seasonal survivors) differed across levels of disturbance and was greatest on our least disturbed study sites \( (\ifmmode\expandafter\bar\else\expandafter\=\fi{x} = 14.00\% ), \) moderate on sites with intermediate levels of disturbance \( (\ifmmode\expandafter\bar\else\expandafter\=\fi{x} = 5.61\% ) \) and zero on highly disturbed sites. On low-disturbance sites, a greater proportion of trans-seasonal survivors were SNV seropositive (28.80%) compared to the intermediate-disturbance sites (16.67). Collectively, our results indicate that habitat disturbance plays a predictive role in SNV prevalence, with highly disturbed sites having reduced long-term survival of deer mice, including survival of infected individuals.




Similar content being viewed by others
References
Abbott KD, Ksiazek TG, Mills JN (1999) Long-term hantavirus persistence in rodent populations in Central Arizona. Emerg Infect Dis 5:102–112
Anderson DC, Harper KT, Rushforth SR (1982) Recovery of cryptogamic soil crusts from grazing on Utah, USA winter ranges. J Range Manage 35:355–359
Biggs JR, Bennett KD, Mullen MA, Haarmann TK, Salisbury M, Robinson RJ, Keller D, Torrez-Martinez N, Hjelle B (2000) Relationship of ecological variables to Sin Nombre virus antibody seroprevalence in populations of deer mice. Ecology 81:676–682
Boone JD, Otteson EW, McGwire KC, Villard P, Rowe JE, St. Jeor SC (1998) Ecology and demographics of hantavirus infections in rodent populations in the Walker River Basin Nevada California. Am J Trop Med Hyg 59:445–451
Borucki MK, Boone JD, Rowe JE, Bohlman MC, Kuhn EA, DeBacca R, St. Jeor SC (2000) Role of maternal antibody in natural infection of Peromyscus maniculatus with Sin Nombre virus. J Virol 74:2426–2429
Botten JK, Mirowsky D, Kusewitt M, Bharadwaj J, Yee R, Ricci RM, Feddersen R, Hjelle B (2000) Experimental infection model for Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus). Proc Natl Acad Sci 97:10578–10583
Botten J, Mirowsky K, Kusewitt D, Ye CY, Gottlieb K, Prescott J, Hjelle B (2003) Persistent Sin Nombre virus infection in the deer mouse (Peromyscus maniculatus) model: sites of replication and strand-specific expression. J Virol 77:1540–1550
Calisher CH, Sweeney W, Mills JN, Beaty BJ (1999) Natural history of Sin Nomber virus in western Colorado. Emerg Infect Dis 5:126–134
Calisher CH, Mills JN, Sweeney WP, Choate JR, Sharp DE, Cannestorp KM, Beaty BJ (2001) Do unusual site-specific population dynamics of rodent reservoirs provide clues to the natural history of hantaviruses? J Wildl Dis 37:280–288
Calisher CH, Root JJ, Mills JM, Beaty BJ (2002) Assessment of ecologic and biologic factors leading to hantavirus pulmonary syndrome, Colorado, USA. Croat Med J 43:330–337
Canfield RH (1941) Application of the line interception method in sampling range vegetation. J For 39:288–304
Centers for Disease Control, Prevention (1995) Methods for trapping and sampling small mammals for virologic testing. Centers for Disease Control, Prevention
Childs JE, Korch GW, Glass GE, De Luc JW, Shah KV (1987) Epizoology of hantavirus infections in Baltimore: characteristics of infected rat populations. Am J Epidemiol 126:55–68
Cnaan A, Laird NM, Slasor P (1997) Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 16:2349–2380
Cole DN (1990) Trampling disturbance and recovery of cryptogamic soil crusts in Grand Canyon National Park Arizona, USA. Great Basin Nat 50:321–326
Douglass RJ, Wilson T, Semmens WJ, Zanto SN, Bond CW, Van Horn RC, Mills JN (2001) Longitudinal studies of Sin Nombre virus in deer mouse-dominated ecosystems of Montana. Am J Trop Med Hyg 65:33–41
Doyle TJ, Bryan RT, Peters CJ (1998) Viral hemorrhagic fever and hantavirus infections in the Americas. Infect Dis Clin North Am 12:95
Feldman H, Sanchez A, Murozonov S, Spiropoulou CF, Rollin PE, Ksiazek TG et al (1993) Utilzation of autopsy RNA for the synthesis of the nucleocapsid antigen of a newly recognized virus associated with hantavirus pulmonary syndrome. Virus Res 30:351–367
Glass GE, Livingstone W, Mills JN, Hlady WJ, Fine JB, Rolin PE et al (1998) Black Creek Canal virus infection in Sigmodon hispidus in southern Florida. Am J Trop Med Hyg 59:699–703
Glass GE, Cheek JE, Patz JA, Shields TM, Doyle TJ, Thoroughman DA, Hunt DK, Enscore RE, Gage KL, Irland C, Peters CJ, Bryan R (2000) Using remotely sensed data to identify areas at risk for hantavirus pulmonary syndrome. Emerg Infect Dis 6:238–247
Hinson ER, Shone SM, Zink MC, Glass GE, Klein SL (2004) Wounding: the primary mode of Seoul Virus transmission among male Norway rats. Am J Trop Med Hyg 70:310–317
Hirst RA, Pywell RF, Marrs RH, Putwain PD (2003) The resistance of a chalk grassland to disturbance. J Appl Ecol 40:368–379
Hjelle B, Jenison S, Torrez-Martines N, Yamada T, Nolte K, Zumwalt R, Macinnes K, Myers G (1994) A novel hantavirus associated with an outbreak of fatal respiratory disease in the southwestern United States; evolutionary relationships to known hantaviruses. J Virol 68:592–596
Johansen JR, St. Clair LL, Webb BL, Nebeker GT (1984) Recovery patterns of cryptogamic soil crusts in desert rangelands following fire disturbance. Bryologist 87:238–243
Johnson PT, Chase JM (2004) Parasites in the food web: linking amphibian malformations and aquatic eutrophication. Ecol Lett 7:521–526
Kilpatrick ED, Terajima M, Koster FT, Catalina MD, Cruz J, Ennis FA (2004) Role of specific CD8+ T cells in the severity of a fulminant zoonotic viral hemorrhagic fever. J Immunol 172:3297–3304
Klein SL, Zink MC, Glass GE (2004) Seoul virus increases aggressive behaviour in male Norway rats. Anim Behav 67:421–429
Kuenzi AJ, Morrison ML, Swann DE, Hardy PC, Downard GT (1999) A longitudinal study of Sin Nombre virus prevalence in rodents, southeastern Arizona. Emerg Infect Dis 5:113–118
Kuenzi AJ, Douglass RJ, Bond CW, Calisher CH, Mills JN (2005) Long-term dynamics of Sin Nombre viral RNA and antibody in deer mice in Montana. J Wildl Dis 41:473–481
Lafferty KD (1997) Environmental parasitology: what can parasites tell us about human impacts on the environment? Parasitol Today 13:251–255
Lafferty KD, Kuris AM (2005) Parasitism and environmental disturbances. In: Thomas F, Renaud F, Guégan JF (eds) Parasitism and ecosystems. Oxford biology. Oxford University Press, Oxford
Langlois JP, Fahrig L, Merriam G, Artsob H (2001) Landscape structure influences continental distribution of hantavirus in deer mice. Landsc Ecol 16:255–266
Long SJ, Blahna DJ (2001) Results of a statewide OHV owner telephone survey for the Little Sahara Recreation Area and surrounding lands. Utah State University professional report IORT PR2001-5
Mackelprang R, Dearing MD, St. Jeor S (2001) High prevalence of Sin Nombre virus in rodent populations, central Utah: a consequence of human disturbance? Emerg Infect Dis 7:480–481
Marcogliese DJ, Ball M, Lankester MW (2001) Potential impacts of clearcutting on parasites of minnows in small boreal lakes. Folia Parasitol 48:269–271
Mills JN, Ksiazek TG, Ellis BA, Rollin PE, Nichol ST, Yates TL,Gannon WL, Levy CE, Engelthaler DM, Davis T, Tanda DT, Frampton W, Nichols CR, Peters CJ, Childs JE (1997) Patterns of association with host and habitat: antibody reactive with Sin Nombre virus in small mammals in the major biotic communities of the southwestern United States. Am J Trop Med Hyg 56:273–284
Mills JN, Johnson JM, Ksiazek TG, Ellis BA, Rollin PE, Yates TL, Mann MO, Johnson MR, Campbell ML, Miyashiro J, Patrick M, Zyzak M, Lavender D, Novak MG, Schmidt K, Peters CJ, Childs JE (1998) A survey of hantavirus antibody in small-mammal populations in selected United States National Parks. Am J Trop Med Hyg 58:525–532
Mills JN, Ksiazek TG, Peters CJ, Childs JE (1999a) Long-term studies of hantavirus reservoir populations in the southwestern United States. A synthesis. Emerg Infect Dis 5:135–142
Mills JN, Yates TL, Ksiazek TG, Peters CJ, Childs JE (1999b) Long-term studies of hantavirus reservoir populations in the southwestern United States: rationale, potential, and methods. Emerg Infect Dis 5:95–101
Netski D, Thran BH, St. Jeor SC (1999) Sin Nombre virus pathogenesis in Peromyscus maniculatus. J Virol 73:585–591
Nicklassson B, Hornfeldt B, Lundkvist A, Bjorsten S, DeLuc J (1995) Temporal dynamics of Puumala virus antibody prevalence in voles and of Nephropathia epidemica incidence in humans. Am J Trop Med Hyg 53:134–140
O’Connor CS, Hayes JP, St. Jeor S (1997) Sin Nombre virus does not impair respiratory function of wild deer mice. J Mammal 78:661–668
Otteson EW, Riolo J, Rowe JE, Nichol ST, Ksiazek TG, Rollin PE, St. Jeor SC (1996) Occurrence of hantavirus within the rodent population of northeastern California and Nevada. Am J Trop Med Hyg 54:127–133
Parmenter CA, Yates TL, Parmenter RR, JL Dunnum (1999) Statistical sensitivity for detection of spatial and temporal patterns in rodent population densities. Emerg Infect Dis 5:118–125
Rowe JE, St. Jeor SC, Riolo J, Otteson EW, Monroe MC, Henderson WW, Ksiazek TG, Rollin PE, Nichol ST (1995) Coexistence of several novel hantaviruses in rodents indigenous to North America. Virology 213:122–130
Smith CB, Urness PJ (1984) Small mammal abundance on native and improved foothill ranges, Utah, USA. J Range Manage 37:353–357
SYSTAT, version 10 (2000) SPSS
Thomas L, Laake JL, Strindberg S, Marques FFC, Buckland ST, Borchers DL, Anderson DR, Burnham KP, Hedley SL, Pollard JH, Bishop JRB (2004) Distance 4.1. Release 2. Research Unit for Wildlife Population Assessment, University of St. Andrews, UK. http://www.ruwpa.st-and.ac.uk/distance/
Wasserberg G, Abramsky Z, Kotler BP, Ostfeld RS, Yarom I, Warburg A (2003) Anthropogenic disturbances enhance occurrence of cutaneous leishmaniasis in Israel deserts: patterns and mechanisms. Ecol Appl 13:868–881
Webb RH (1983) Compaction of desert soils by off-road vehicles. In: Webb RH, Wilshire HG (eds) Environmental effects of off-road vehicles: impacts and management in arid regions. Springer, Berlin Heidelberg New York, pp 51–79
Yamada T, Hjelle B, Lanzi R, Morris C, Anderson B, Jensen S (1995) Antibody responses to four corners hantavirus infection in the deer mouse (Peromyscus maniculatus): identification of an immunodominant region of the viral nucleocapsid protein. J Virol 69:1939–1943
Acknowledgements
Research support was provided by a NSF-NIH grant (EF 0326999) and University of Utah seed grant to M. D. D.; an NIH Training Grant to E. M. L. (AI055434-01A1), a University of Utah Graduate Research Fellowship to C. A. C.; an NSF Predoctoral Fellowship, Utah Wildlife Society, American Society of Mammalogists, and Society for Integrative and Comparative Biology to J. P.-D. We thank J. Allen, S. Appleby, J. Billy, J. Kendrick, C. Votaw, B. Wood, and numerous undergraduate students for assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Craig Osenberg.
Rights and permissions
About this article
Cite this article
Lehmer, E.M., Clay, C.A., Pearce-Duvet, J. et al. Differential regulation of pathogens: the role of habitat disturbance in predicting prevalence of Sin Nombre virus. Oecologia 155, 429–439 (2008). https://doi.org/10.1007/s00442-007-0922-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0922-9