Abstract
Background
Ventral hernia is a commonly occurring surgical problem. Our earlier studies have shown that a 30 mg/kg dose of doxycycline can significantly impact the strength of polypropylene (PP) mesh in a rat hernia repair model at 6 and 12 weeks. The objective of the present study was to investigate the dose dependence of doxycycline treatment on hernia repair strengths in rats.
Study design
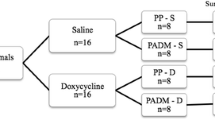
Fifty-six Sprague-Dawley rats underwent hernia repair with either PP mesh (n = 28) or sutures only (primary; n = 28); both groups were further divided into four doxycycline groups of seven animals each: control (0 mg/kg), low (3 mg/kg), medium (10 mg/kg), and high (30 mg/kg). One day before hernia repair surgery, animals received doxycycline doses by gavage and continued receiving daily until euthanasia. After 8 weeks, rats were euthanized and tissue samples from hernia repaired area were collected and analyzed for tensile strength using a tensiometer (Instron, Canton, MA, USA), while MMPs 2, 3, and 9, and collagen type 1 and 3 were analyzed by western blotting.
Results
In mesh-repaired animals, medium and high doxycycline dose repaired mesh fascia interface (MFI) showed significant increase in tensile strength when compared to control. In the primary repaired animals, there was no significant difference in MFI tensile strength in any dose group. In medium-dose MFI, there was a significant reduction in MMPs 2, 3, and 9. In this animal group, MFI showed significant increase in collagen 1 and significant reduction in collagen type 3 when compared to control.
Conclusion
It is possible to improve the strength of mesh-repaired tissue by administering a significantly lower dose of the drug, which has implications for translation of the findings.








Similar content being viewed by others
References
Rutkow IM, Robbins AW (1993) Demographic, classificatory, and socioeconomic aspects of hernia repair in the United States. Surg Clin N Am 73:413–426
Kingsnorth A (2006) The management of incisional hernia. Ann R Coll Surg Engl 88:252–260
Mudge M, Hughes LE (1985) Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg 72:70–71
George CD, Ellis H (1986) The results of incisional hernia repair: a twelve year review. Ann R Coll Surg Engl 68:185–187
Langer S, Christiansen J (1985) Long-term results after incisional hernia repair. Acta Chir Scand 151:217–219
Anthony T, Bergen PC, Kim LT (2000) Factors affecting recurrence following incisional herniorrhaphy. World J Surg 24:95–101
Leber GE, Garb JL, Alexander AJ, Reed WP (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg 133:378–382
Franz MG (2008) The biology of hernia formation. Surg Clin N Am 88:1–15
Salameh JR, Talbott LM, May W, Gosheh B, Vig PJ, McDaniel DO (2007) Role of biomarkers in incisional hernias. Am Surg 73:561–567; discussion 567–568
Antoniou SA, Antoniou GA, Granderath FA, Simopoulos C (2009) The role of matrixmetallo-proteinases in the pathogenesis of abdominal wall hernias. Eur J Clin Invest 39:953–959
Lee HM, Ciancio SG, Tüter G, Ryan ME, Komaroff E, Golub LM (2004) Subantimicrobial dose doxycycline efficacy as a matrix metalloproteinase inhibitor in chronic periodontitis patients is enhanced when combined with a non-steroidal antiinflammatory drug. J Periodontol 75:453–463
Tharappel JC, Bower CE, Whittington-Harris J, Ramineni SK, Puleo DA, Roth JS (2014) Doxycycline administration improves fascial interface in hernia repair. J Surg Res 190:692–698
Tharappel JC, Ramineni SK, Reynolds D, Puleo DA, Roth JS (2013) Doxycycline impacts hernia repair outcomes. J Surg Res 184:699–704
Rice RD, Ayubi FS, Shaub ZJ, Parker DM, Armstrong PJ, Tsai JW (2010) Comparison of Surgisis, AlloDerm, and Vicryl woven mesh grafts for abdominal wall defect repair in an animal model. Aesth Plast Surg 34:290–296
White AC, Hayat CS, Kimball KT (1987) Effects of lipophilic tetracyclines against Giardia lamblia. J Infect Dis 28:781–787
Sezaki M, Inouye S, Kondo S (1992) A new tetracycline antibiotic with antitumor activity. I. Taxonomy and fermentation of the producing strain, isolation and characterization of SF-2575. J Antibiotics 45:320–324
Flanigan TP, Soave R (1991) Plasmodium falciparum growth and the effects of chlortetracycline. Clin Infect Dis 63:388–392
Castro MM, Tanus-Santos JE (2013) Inhibition of matrix metalloproteinases (MMPs) as a potential strategy to amelioratehypertension-induced cardiovascular alterations. Curr Drug Targets 14:335–343
Antonio RC, Ceron CS, Rizzi E, Coelho EB, Tanus-Santos JE, Gerlach RF (2014) Antioxidant effect of doxycycline decreases MMP activity and blood pressure in SHR. Mol Cell Biochem 386:99–105
Wysocki AB, Staiano-Coico L, Grinnell F (1993) Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol 101:64–68
Woessner JF (1994) The family of matrix metalloproteinases. Ann N Y Acad Sci 732:11–21
Mignatti P, Rifkin DB, Welgus HG, Parks WC (1996) Proteinases and tissue remodeling. In: Clark RAF (ed) The molecular and cellular biology of wound repair, 2nd edn. Plenum Press, New York, pp 427–474
Murphy G, Willenbrock F, Crabbe T, O’Shea M, Ward R, Atkinson S, O’Connell J, Docherty A (1994) Regulation of matrix metalloproteinase activity. Ann N Y Acad Sci 732:31–41
Smith APS (2003) The role of MMPs in chronic wound edema. Podiatry Today 16:22–26
Wilcox JR, Covington DS, Paez N (2012) Doxycycline as a modulator of inflammation in chronic wounds. Wounds 24:339–349
Ryan ME, Ashley RA (1998) How do tetracyclines work? Adv Dent Res 12:149–151
Lorenzl S, Albers DS, Narr S, Chirichigno J, Beal MF (2002) Expression of MMP-2, MMP-9, and MMP-1 and their endogenous counterregulators TIMP-1 and TIMP-2 in postmortem brain tissue of Parkinson’s disease. Exp Neurol 178:13–20
Guedez L, Courtemanch L, Stetler-Stevenson M (1998) Tissue inhibitor of metalloproteinase (TIMP)-1 induces differentiation and an antiapoptotic phenotype in germinal center B cells. Blood 92:1342–1349
Guimaraes DA, Rizzi E, Ceron CS, Oliveira AM, Oliveira DM, Castro MM, Tirapelli CR, Gerlach RF, Tanus-Santos JE (2011) Doxycycline dose dependently inhibits MMP-2-mediated vascular changes in 2K1C hypertension. Basic Clin Pharmacol Toxicol 108:318–325
Nagase N (2001) Substrate specificity of MMPs. In: Clendeninn NJ, Krzysztof A (eds) Cancer drug discovery and development: matrix metalloproteinase inhibitors in cancer therapy. Humana Press Inc., Totowa
Acknowledgments
This research was supported by a grant from Davol, Inc., Warwick, Rhode Island. Timely help in the preparation and submission of the manuscript by Ms. Linda Combs is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Tharappel, Harris, Zwischenberger, and Levy have no conflicts of interest or financial ties to disclose. Dr. Puleo receives Grant funding from the NIH (R01AR060964). Dr. Roth has received external grant funding from Davol, Inc. for the support of this study; he has also received external grant funding and consulting fees from CR Bard; he is on speaker bureaus for CR Bard and Ethicon; and he is a Board member of the Musculoskeletal Transplant Foundation.
Rights and permissions
About this article
Cite this article
Tharappel, J.C., Harris, J.W., Zwischenberger, B.A. et al. Doxycycline shows dose-dependent changes in hernia repair strength after mesh repair. Surg Endosc 30, 2016–2021 (2016). https://doi.org/10.1007/s00464-015-4434-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4434-0