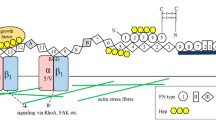
Abstract
Fibronectin (FN) is a cell adhesion protein that binds integrins in a process also involving the protein-crosslinking enzyme transglutaminase 2 (TG2) as a co-receptor. The cell-adhesive property of TG2 has been linked to a complex formation with FN and to its ability to crosslink and polymerize FN on the cell surface. We tested here the effects of extracellular FN, before and after in vitro crosslinking and polymerization by TG2, on MC3T3-E1 osteoblast adhesion. We show that TG2-mediated crosslinking creates large, compacted chain-like protein clusters that include both TG2 and FN molecules as analyzed by Western blotting and atomic force microscopy. Crosslinking of FN significantly promotes osteoblast adhesion as measured by crystal violet staining, and enhances β1-integrin clustering on the cell surface as visualized by immunofluorescence microscopy. We hypothesize that TG2-mediated crosslinking enhances the cell-adhesive properties of FN by increasing the molecular rigidity of FN in the extracellular matrix.



Similar content being viewed by others
References
Akimov SS, Krylov D, Fleischman LF, Belkin AM (2000) Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol 148:825–838
Al-Jallad HF, Nakano Y, Chen JL, McMillan E, Lefebvre C, Kaartinen MT (2006) Transglutaminase activity regulates osteoblast differentiation and matrix mineralization in MC3T3-E1 osteoblast cultures. Matrix Biol 25:135–148
Barry ELR, Mosher DF (1988) Factor XIII cross-linking of fibronectin at cellular matrix assembly sites. J Biol Chem 263:10464–10469
Chau DY, Collighan RJ, Verderio EA, Addy VL, Griffin M (2005) The cellular response to transglutaminase-crosslinked collagen. Biomaterials 26:6518–6529
Choquet D, Felsenfeld DP, Sheetz MP (1997) Extracellular matrix rigidity causes strenghtening of inegrin-cytoskeletal linkages. Cell 88:39–48
Engler AJ, Griffin MA, Sen S, Bonneman CG, Sweeny HL, Dischler DE (2004) Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 166:877–887
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126:677–689
Gaudry CA, Vederio E, Aeschlimann D, Cox A, Smith C, Griffin M (1999) Cell surface localization of tissue transglutaminase independent on a fibronectin-binding site in its N-terminal β-sandwich domain. J Biol Chem 274:30707–30714
Geiger B, Bershadsky A, Pankov R, Yamada KM (2001) Transmembrane extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2:793–805
Gentile V, Thomazy V, Piacentini M, Fesus L, Davies PJ (1992) Expression of tissue transglutaminase in Balb-C 3T3 fibroblasts: effects on cellular morphology and adhesion. J Cell Biol 119:463–474
Heath DJ, Downes S, Verderio E, Griffin M (2001) Characterization of tissue transglutaminase in human osteoblast-like cells. J Bone Miner Res 16:1477–1485
Janiak A, Zemskov EA, Belkin AM (2006) Cell surface transglutaminase promotes RhoA activation via integrin clustering and suppression of the Src-p190PhoGAP signaling pathway. Mol Biol Cell 17:1606–1619
Jiang G, Huang AH, Cai Y, Tanase M, Sheetz MP (2006) Rigidity sensing at the leading edge through integrins and RPTPα. Biophys J 90:1804–1809
Jones RA, Nicholas B, Mian S, Davies PJ, Griffin M (1997) Reduced expression of tissue transglutaminase in a human endothelial cell line leads to changes in cell spreading, cell adhesion and reduced polymerization of fibronectin. J Cell Sci 110:2461–2472
Kaartinen MT, El-Maadawy S, Räsänen NH, McKee MD (2002) Transglutaminase and its substrates in bone. J Bone Miner Res 12:2161–2173
Kaartinen MT, Sun W, Kaipathur N, McKee MD (2005) Transglutaminase crosslinking of SIBLING proteins in teeth. J Dent Res 84:607–612
Kostic A, Sheetz MP (2006) Fibronectin rigidity response though Fyn and p130Cas recruitment to the leading edge. Mol Niol Cell 17:2684–2695
Lorand L, Graham RM (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4:140–156
Martinez J, Chalupowicz DG, Roush RK, Sheth A, Barsigian C (1994) Transglutaminase-mediated processing of fibronectin by endothelial cell monolayers. Biochemistry 33:2538–2545
Moursi AM, Damsky CH, Lull J, Zimmerman D, Doty SB, Aota S, Globus RK (1996) Fibronectin regulates calvarial osteoblast differentiation. J Cell Sci 109:1369–1380
Nelea V, Nakano Y, Kaartinen MT (2008) Size distribution and molecular association of plasma fibronectin and fibronectin crosslinked with transglutaminase 2. Protein J 27:223–233
Pankov R, Yamada KM (2002) Fibronectin at a glance. J Cell Sci 115:3861–3863
Sechler JL, Schwarzbauer JE (1998) Control of cell cycle progression by fibronectin matrix architecture. J Biol Chem 273:25533–25536
Tang C, Yang R, Huang T, Liu S, Fu W (2004) Enhancement of fibronectin fibrillogenesis and bone formation by basic fibroblast growth factor via a protein kinase C-dependent pathway in rat osteoblasts. Mol Pharmacol 66:440–449
Verderio E, Coombes A, Jones RA, Li X, Heath D, Downes S, Griffin M (2001) Role of the cross-linking enzyme tissue transglutaminase in the biological recognition of synthetic biodegradable polymers. J Biomed Mater Res 54:294–304
Verderio E, Nicholas B, Gross S, Griffin M (1998) Regulated expression of tissue transglutaminase in Swiss 3T3 fibroblasts: effect on the processing of fibronectin, cell attachment and cell death. Exp Cell Res 239:119–138
Verderio EA, Telci D, Okoye A, Melino G, Griffin M (2003) A novel RGD-independent cell adhesion pathway mediated by fibronectin-bound tissue transglutaminase rescues cells from anoikis. J Biol Chem 278(43):42604–42614
Wang D, Christensen K, Chawala K, Xiao G, Krebsbach PH, Franceschi RT (1999) Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res 9:843–854
Zhang Q, Mosher DF (1996) Cross-linking of the NH2-terminal region of fibronectin to molecules of large apparent molecular mass. J Biol Chem 271:33284–33292
Acknowledgments
This study was supported by a grant to MTK from the Canadian Institutes of Health Research. MTK is a scholar of the Fonds de la recherche en santé du Québec, and a member of the McGill Center for Bone and Periodontal Research and the McGill Center for Biorecognition and Biosensors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forsprecher, J., Wang, Z., Nelea, V. et al. Enhanced osteoblast adhesion on transglutaminase 2-crosslinked fibronectin. Amino Acids 36, 747–753 (2009). https://doi.org/10.1007/s00726-008-0125-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0125-7