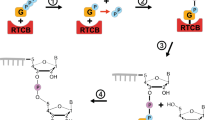
Abstract
The unusual basic amino acid, hypusine [Nε-(4-amino-2-hydroxybutyl)-lysine], is a modified lysine with the addition of the 4-aminobutyl moiety from the polyamine spermidine. This naturally occurring amino acid is a product of a unique posttranslational modification that occurs in only one cellular protein, eukaryotic translation initiation factor 5A (eIF5A, eIF-5A). Hypusine is synthesized exclusively in this protein by two sequential enzymatic steps involving deoxyhypusine synthase (DHS) and deoxyhypusine hydroxylase (DOHH). The deoxyhypusine/hypusine synthetic pathway has evolved in archaea and eukaryotes, and eIF5A, DHS and DOHH are highly conserved suggesting a vital cellular function of eIF5A. Gene disruption and mutation studies in yeast and higher eukaryotes have provided valuable information on the essential nature of eIF5A and the deoxyhypusine/hypusine modification in cell growth and in protein synthesis. In view of the extraordinary specificity and functional significance of hypusine-containing eIF5A in mammalian cell proliferation, eIF5A and the hypusine biosynthetic enzymes are novel potential targets for intervention in aberrant cell proliferation.




Similar content being viewed by others
Abbreviations
- aIF5A:
-
Archaeal initiation factor 5A
- EF-P:
-
Elongation factor P
- eIF5A:
-
Eukaryotic translation initiation factor 5A
- eIF5A-1:
-
Primary isoform of eIF5A
- eIF5A-2:
-
Secondary isoform of eIF5A
- eIF5A(Lys):
-
eIF5A precursor
- eIF5A(Dhp):
-
eIF5A intermediate containing deoxyhypusine
- eIF5A(Hpu):
-
eIF5A active form containing hypusine
- UBR5A:
-
Ubiquitin arginine-fusion yeast eukaryotic initiation factor 5A
- DHS:
-
Deoxyhypusine synthase
- DOHH:
-
Deoxyhypusine hydroxylase
- GC7:
-
N1-guanyl-1,7-diaminoheptane
- A site:
-
Aminoacyl-tRNA site
- P site:
-
Peptidyl-tRNA site
- E site:
-
Exiting tRNA site
References
Abbruzzese A, Park MH, Folk JE (1986) Deoxyhypusine hydroxylase from rat testis: partial purification and characterization. J Biol Chem 261:3085–3089
Aoki H, Xu J, Emili A, Chosay JG, Golshani A, Ganoza MC (2008) Interactions of elongation factor EF-P with the Escherichia coli ribosome. FEBS J 275:671–681
Balasundaram D, Tabor CW, Tabor H (1991) Spermidine or spermine is essential for the aerobic growth of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 88:5872–5876
Bartig D, Schümann H, Klink F (1990) The unique posttranslational modification leading to deoxyhypusine or hypusine is a general feature of the Archebacterial kingdom. System Appl Microbiol 13:112–116
Bevec D, Kappel B, Jaksche H, Csonga R, Hauber J, Klier H, Steinkasserer A (1996) Molecular characterization of a cDNA encoding functional human deoxyhypusine synthase and chromosomal mapping of the corresponding gene locus. FEBS Lett 378:195–198
Blaha G, Stanley RE, Steitz TA (2009) Formation of the first peptide bond: the structure of EF-P bound to the 70s ribosome. Science 325:966–970
Brochier C, Lopez-Garcia P, Moreira D (2004) Horizontal gene transfer and archaeal origin of deoxyhypusine synthase homologous genes in bacteria. Gene 330:169–176
Byers TL, Lakanen JR, Coward JK, Pegg AE (1994) The role of hypusine depletion in cytostasis induced by S-adenosyl-L-methionine decarboxylase inhibition: new evidence provided by L-methylspermidine and 1,12-dimethylspermine. Biochem J 303:363–368
Cano VS, Jeon GA, Johansson HE, Henderson CA, Park JH, Valentini SR, Hershey JW, Park MH (2008) Mutational analyses of human eIF5A-1—identification of amino acid residues critical for eIF5A activity and hypusine modification. FEBS J 275:44–58
Chatterjee I, Gross SR, Kinzy TG, Chen KY (2006) Rapid depletion of mutant eukaryotic initiation factor 5A at restrictive temperature reveals connections to actin cytoskeleton and cell cycle progression. Mol Genet Genomics 275:264–276
Chattopadhyay MK, Tabor CW, Tabor H (2003) Spermidine but not spermine is essential for hypusine biosynthesis and growth in Saccharomyces cerevisiae: spermine is converted to spermidine in vivo by the fms1-amine oxidase. Proc Natl Acad Sci USA 100:13869–13874
Chattopadhyay MK, Park MH, Tabor H (2008) Hypusine modification for growth is the major function of spermidine in Saccharomyces cerevisiae polyamine auxotrophs grown in limiting spermidine. Proc Natl Acad Sci USA 105:6554–6559
Chen KY, Liu AY (1997) Biochemistry and function of hypusine formation on eukaryotic initiation factor 5A. Biol Signals 6:105–109
Clement PC, Henderson CA, Jenkins ZA, Smit-McBride Z, Wolff EC, Hershey JWB, Park MH, Johansson HE (2003) Identification and characterization of eukaryotic initiation factor 5A-2. Eur J Biochem 147:4254–4263
Dias CA, Cano VS, Rangel SM, Apponi LH, Frigieri MC, Muniz JR, Garcia W, Park MH, Garratt RC, Zanelli CF, Valentini SR (2008) Structural modeling and mutational analysis of yeast eukaryotic translation initiation factor 5A reveal new critical residues and reinforce its involvement in protein synthesis. FEBS J 275:1874–1888
Frigieri MC, Thompson GM, Pandolfi JR, Zanelli CF, Valentini SR (2007) Use of a synthetic lethal screen to identify genes related to TIF51a in Saccharomyces cerevisiae. Genet Mol Res 6:152–165
Frigieri MC, Joao Luiz MV, Apponi LH, Zanelli CF, Valentini SR (2008) Synthetic lethality between eIF5A and Ypt1 reveals a connection between translation and the secretory pathway in yeast. Mol Genet Genomics 280:211–221
Glick BR, Ganoza MC (1975) Identification of a soluble protein that stimulates peptide bond synthesis. Proc Natl Acad Sci USA 72:4257–4260
Gregio AP, Cano VP, Avaca JS, Valentini SR, Zanelli CF (2009) eIF5A has a function in the elongation step of translation in yeast. Biochem Biophys Res Commun 380:785–790
Guan XY, Sham JS, Tang TC, Fang Y, Huo KK, Yang JM (2001) Isolation of a novel candidate oncogene within a frequently amplified region at 3q26 in ovarian cancer. Cancer Res 61:3806–3809
Guan XY, Fung JM, Ma NF, Lau SH, Tai LS, Xie D, Zhang Y, Hu L, Wu QL, Fang Y, Sham JS (2004) Oncogenic role of eIF-5A2 in the development of ovarian cancer. Cancer Res 64:4197–4200
Hanauske-Abel HM, Park MH, Hanauske AR, Popowicz AM, Lalande M, Folk JE (1994) Inhibition of the G1-S transition of the cell cycle by inhibitors of deoxyhypusine hydroxylation. Biochim Biophys Acta 1221:115–124
Hanawa-Suetsugu K, Sekine S, Sakai H, Hori-Takemoto C, Terada T, Unzai S, Tame JR, Kuramitsu S, Shirouzu M, Yokoyama S (2004) Crystal structure of elongation factor P from Thermus thermophilus hb8. Proc Natl Acad Sci USA 101:9595–9600
Hanazawa M, Kawasaki I, Kunitomo H, Gengyo-Ando K, Bennett KL, Mitani S, Iino Y (2004) The Caenorhabditis elegans eukaryotic initiation factor 5A homologue, IFF-1, is required for germ cell proliferation, gametogenesis and localization of the P-granule component PGL-1. Mech Dev 121:213–224
Igarashi K, Kashiwagi K (2009) Modulation of cellular function by polyamines. Int J Biochem Cell Biol (in press)
Jansson BP, Malandrin L, Johansson HE (2000) Cell cycle arrest in archaea by the hypusination inhibitor N(1)-guanyl-1,7-diaminoheptane. J Bacteriol 182:1158–1161
Jao DL, Chen KY (2006) Tandem affinity purification revealed the hypusine-dependent binding of eukaryotic initiation factor 5A to the translating 80s ribosomal complex. J Cell Biochem 97:583–598
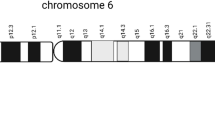
Jenkins ZA, Haag PG, Johansson HE (2001) Human eIF5A2 on chromosome 3q25-q27 is a phylogenetically conserved vertebrate variant of eukaryotic translation initiation factor 5A with tissue-specific expression. Genomics 71:101–109
Joe YA, Wolff EC, Park MH (1995) Cloning and expression of human deoxyhypusine synthase cDNA. Structure–function studies with the recombinant enzyme and mutant proteins. J Biol Chem 270:22386–22392
Kang HA, Hershey JW (1994) Effect of initiation factor 5A depletion on protein synthesis and of Saccharomyces cerevisiae. J Biol Chem 269:3934–3940
Kemper WM, Berry KW, Merrick WC (1976) Purification and properties of rabbit reticulocyte protein synthesis initiation factors M2Balpha and M2Bbeta. J Biol Chem 251:5551–5557
Maeda I, Kohara Y, Yamamoto M, Sugimoto A (2001) Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr Biol 11:171–176
Magdolen V, Klier H, Wohl T, Klink F, Hirt H, Hauber J, Lottspeich F (1994) The function of the hypusine-containing proteins of yeast and other eukaryotes is well conserved. Mol Gen Genet 244:646–652
Park MH (2006) The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J Biochem (Tokyo) 139:161–169
Park MH, Wolff EC, Lee YB, Folk JE (1994) Antiproliferative effects of inhibitors of deoxyhypusine synthase. Inhibition of growth of Chinese hamster ovary cells by guanyl diamines. J Biol Chem 269:27827–27832
Park MH, Lee YB, Joe YA (1997) Hypusine is essential for eukaryotic cell proliferation. Biol Signals 6:115–123
Park MH, Joe YA, Kang KR (1998) Deoxyhypusine synthase activity is essential for cell viability in the yeast Saccharomyces cerevisiae. J Biol Chem 273:1677–1683
Park J-H, Aravind L, Wolff EC, Kaevel J, Kim YS, Park MH (2006) Molecular cloning, expression, and structural prediction of deoxyhypusine hydroxylase: a HEAT-repeat-containing metalloenzyme. Proc Natl Acad Sci USA 103:51–56
Parker R, Sheth U (2007) P bodies and the control of mRNA translation and degradation. Mol Cell 25:635–646
Patel PH, Costa-Mattiolo M, Schulze KL, Bellen HJ (2009) The Drosophila deoxyhypusine hydroxylase homologue nero and its target eIF5A are required for cell growth and the regulation of autophagy. J Cell Biol 185:1181–1194
Peltz SW, Donahue JL, Jacobson A (1992) A mutation in the tRNA nucleotidyltransferase gene promotes stabilization of mRNAs in Saccharomyces cerevisiae. Mol Cell Biol 12:5778–5784
Saini P, Eyler DE, Green R, Dever TE (2009) Hypusine-containing protein eIF5A promotes translation elongation. Nature 459:118–121
Sasaki K, Abid MR, Miyazaki M (1996) Deoxyhypusine synthase gene is essential for cell viability in the yeast Saccharomyces cerevisiae. FEBS Lett 384:151–154
Schnier J, Schwelberger HG, Smit-McBride Z, Kang HA, Hershey JW (1991) Translation initiation factor 5A and its hypusine modification are essential for viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol 11:3105–3114
Schrader R, Young C, Kozian D, Hoffmann R, Lottspeich F (2006) Temperature-sensitive eIF5A mutant accumulates transcripts targeted to the nonsense-mediated decay pathway. J Biol Chem 281:35336–35346
Schwelberger HG, Kang HA, Hershey JW (1993) Translation initiation factor eIF-5A expressed from either of two yeast genes or from human cDNA. Functional identity under aerobic and anaerobic conditions. J Biol Chem 268:14018–14025
Shiba T, Mizote H, Kaneko T, Nakajima T, Kakimoto Y (1971) Hypusine, a new amino acid occurring in bovine brain. Isolation and structural determination. Biochim Biophys Acta 244:523–531
Spradling AC, Stern D, Beaton A, Rhem EJ, Laverty T, Mozden N, Misra S, Rubin GM (1999) The Berkeley Drosophila genome project gene disruption project: single P-element insertions mutating 25% of vital Drosophila genes. Genetics 153:135–177
Tao Y, Chen KY (1995) Molecular cloning and functional expression of Neurospora deoxyhypusine synthase cDNA and identification of yeast deoxyhypusine synthase cDNA. J Biol Chem 270:23984–23987
Thompson GM, Cano VS, Valentini SR (2003) Mapping eIF5A binding sites for Dys1 and Lia1: in vivo evidence for regulation of eIF5A hypusination. FEBS Lett 555:464–468
Tong Y, Park I, Hong BS, Nedyalkova L, Tempel W, Park HW (2009) Crystal structure of human eIF5A1: insight into functional similarity of human eIF5A1 and eIF5A2. Proteins 75:1040–1045
Valentini SR, Casolari JM, Oliveira CC, Silver PA, McBride AE (2002) Genetic interactions of yeast eukaryotic translation initiation factor 5A(eIF5A) reveal connections to poly(A)-binding protein and protein kinase C signaling. Genetics 160:393–405
Vu VV, Emerson JP, Martinho M, Kim YS, Munck E, Park MH, Que L Jr (2009) Human deoxyhypusine hydroxylase, an enzyme involved in regulating cell growth, activates O2 with a nonheme diiron center. Proc Natl Acad Sci USA 106:14814–14819
Weir BA, Yaffe MP (2004) Mmd1p, a novel, conserved protein essential for normal mitochondrial morphology and distribution in the fission yeast Schizosaccharomyces pombe. Mol Biol Cell 15:1656–1665
Wöhl T, Klier H, Ammer H (1993) The HYP2 gene of Saccharomyces cerevisiae is essential for aerobic growth: characterization of different isoforms of the hypusine-containing protein Hyp2p and analysis of gene disruption mutants. Mol Gen Genet 241:305–311
Wolff EC, Kang KR, Kim YS, Park MH (2007) Posttranslational synthesis of hypusine: evolutionary progression and specificity of the hypusine modification. Amino Acids 33:341–350
Zanelli CF, Valentini SR (2005) Pkc1 acts through Zds1 and Gic1 to suppress growth and cell polarity defects of a yeast eIF5A mutant. Genetics 171:1571–1581
Zanelli CF, Valentini SR (2007) Is there a role for eIF5A in translation? Amino Acids 33:351–358
Zanelli CF, Maragno AL, Gregio AP, Komili S, Pandolfi JR, Mestriner CA, Lustri WR, Valentini SR (2006) eIF5A binds to translational machinery components and affects translation in yeast. Biochem Biophys Res Commun 348:1358–1366
Zuk D, Jacobson A (1998) A single amino acid substitution in yeast eIF-5A results in mRNA stabilization. EMBO J 17:2914–2925
Acknowledgments
The research was supported in part by the Intramural Research Program of National Institute of Dental and Craniofacial Research (NIDCR), NIH, FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) and KANAE Foundation for the Promotion of Medical Science. We thank Dr. Edith C. Wolff (NIDCR, NIH) for critical reading of the manuscript and helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, M.H., Nishimura, K., Zanelli, C.F. et al. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 38, 491–500 (2010). https://doi.org/10.1007/s00726-009-0408-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-009-0408-7