Abstract
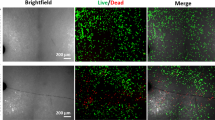
Bone cells are connected to one another in a network, via their dendritic cellular processes. Previously, we hypothesized that these processes could be ruptured by microcracks. We proposed this as a mechanism by which osteoctyes could detect the presence of microcracks. In order for this mechanism to be effective, the number of ruptured processes would have to increase with microcrack length and also with the applied cyclic stress. This paper presents for the first time experimental data, which shows that this is indeed the case. We examined samples of bovine, ovine and murine bone ex vivo and observed processes passing across crack faces: some were still intact whilst others had ruptured. The number of intact processes per unit crack length decreased significantly with increasing crack length and also decreased in samples, which had been tested in vitro at higher stress levels. A theoretical model that we had developed previously was able to predict the overall magnitude and general trends in the experimental data. This work has provided further support for our “scissors” model, which proposes that microcracks can be detected because they disturb the osteocyte network, specifically by rupturing cellular processes where they pass across the crack faces.
Similar content being viewed by others
References
Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB (1998) Intracortical remodeling in adult rat long bones after fatigue loading. Bone 23: 275–281
Burr DB (2002) Targeted and nontargeted remodeling. Bone 30: 2–4
Burr DB, Martin B (1993) Calculating the probability that microcracks initiate resorption spaces. J Biomech 26: 613–616
Chattah NLT, Sharir A, Weiner S, Shahar R (2009) Determining the elastic modulus of mouse cortical bone using electronic speckle pattern interferometry (ESPI) and micro computed tomography: A new approach for characterizing small-bone material properties. Bone 45: 84–90
Currey J (2002) Bones: structure and mechanics. Princeton University Press, USA
Dunstan CR, Somers NM, Evans RA (1993) Osteocyte death and hip fracture. Calcif Tissue Int 53: S113–S118
Frost HM (1960) Presence of microscopic cracks in vivo in bone. Henry Ford Hosp Med Bull 8: 25–35
Han Y, Cowin SC, Schaffler MB, Weinbaum S (2004) Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci USA 101: 16689–16694
Hazenberg JG, Freeley M, Foran E, Lee TC, Taylor D (2006) Microdamage: a cell transducing mechanism based on ruptured osteocyte processes. J Biomech 39: 2096–2103
Klein-Nulend J, Bacabac RG, Mullender MG (2005) Mechanobiology of bone tissue. Pathologie Biologie 53: 576–580
Lee TC, Staines A, Taylor D (2002) Bone adaptation to load: microdamage as a stimulus for bone remodelling. J Anat 201: 437–446
Mori S, Burr DB (1993) Increased intracortical remodeling following fatigue damage. Bone 14: 103–109
Mulcahy LE, Taylor D, Lee TC, Duffy GP (2011) RANKL and OPG activity is regulated by injury size in networks of osteocyte-like cells. Bone 48: 182–188
Mullender MG, Huiskes R, Versleyen H, Buma P (1996) Osteocyte density and histomorphometric parameters in cancellous bone of the proximal femur in five mammalian species. J Orthop Res 14: 972–979
Noble B, Alini M, Richards RG (2003) Bone microdamage and cell apoptosis. Eur Cells Mater 6: 46–55
O’Brien F, Taylor D, Lee TC (2002) An improved labelling technique for monitoring microcrack growth in bone. J Biomech 35: 523–526
Prendergast PJ, Taylor D (1994) Prediction of bone adaptation using damage accumulation. J Biomech 27: 1067–1076
Qiu SJ, Boyce TM, Schaffler MB (1997) Osteocyte loss and microdamage in aging human compact bone. Trans Orthop Res Soc: 88
Schaffler MB, Pitchford WC, Choi K, Riddle JM (1994) Examination of compact bone microdamage using back-scattered electron microscopy. Bone 15: 483–488
Schneider P, Meier M, Wepf R, Müller R (2010) Towards quantitative 3D imaging of the osteocyte lacuno-canalicular network. Bone 47: 848–858
Sugawara Y, Kamioka H, Honjo T, Tezuka KI, Takano-Yamamoto T (2005) Three-dimensional reconstruction of chick calvarial osteocytes and their cell processes using confocal microscopy. Bone 36: 877–883
Tami AE, Nasser P, Verborgt O, Schaffler MB, Knothe Tate ML (2002) The role of interstitial fluid flow in the remodeling response to fatigue loading. J Bone Miner Res 17: 2030–2037
Taylor D, Casolari E, Bignardi C (2004) Predicting stress fractures using a probabilistic model of damage, repair and adaptation. J Orthop Res 22: 487–494
Taylor D, Hazenberg JG, Lee TC (2003) The cellular transducer in damage-stimulated bone remodelling: A theoretical investigation using fracture mechanics. J Theor Biol 225: 65–75
Taylor D, Lee TC (1998) Measuring the shape and size of microcracks in bone. J Biomech 31: 1177–1180
Temiyasathit S, Jacobs CR (2010) Osteocyte primary cilium and its role in bone mechanotransduction. Ann N Y Acad Sci 1192: 422–428
Verborgt O, Gibson GJ, Schaffler MB (2000) Loss of osteocyte integrity in association with microdamage and bone remodelling after fatigue in vivo. J Bone Miner Res 15: 60–67
Yamada H (1970) Strength of biological materials. Williams and Wilkins, Baltimore, USA
You J, Yellowley CE, Donahue HJ, Zhang Y, Chen Q, Jacobs CR (2000) Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng 122: 387–393
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dooley, C., Tisbo, P., Lee, T.C. et al. Rupture of osteocyte processes across microcracks: the effect of crack length and stress. Biomech Model Mechanobiol 11, 759–766 (2012). https://doi.org/10.1007/s10237-011-0349-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-011-0349-4