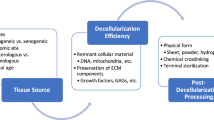
Abstract
Biologic scaffold materials composed of mammalian extracellular matrix (ECM) are prepared by decellularization of source tissues harvested from either humans (allogeneic) or a variety of other (xenogeneic) species. These matrix scaffold materials are commonly regulated and used as surgical mesh materials for applications such as ventral hernia repair, musculotendinous tissue reconstruction, dura mater replacement, reconstructive breast surgery, pelvic floor reconstruction, and the treatment of cutaneous ulcers, among others. The clinical results for these applications vary widely for reasons which include characteristics of the source tissue, methods and efficacy of tissue decellularization, and methods of processing/manufacturing. However, the primary determinant of success or failure in the clinical setting is the response of the host to these implanted biologic scaffold materials. It is logical to question why any non-self biologic material, particularly a xenogeneic material, would not elicit an early and aggressive adverse immune response. The present manuscript briefly describes the known mechanisms by which these biologic scaffold materials can facilitate a constructive remodeling response, the known causative factors of an adverse response, and provides a general discussion of the role of the macrophage in determining outcome.
Similar content being viewed by others
Abbreviations
- DAMP:
-
Damage associated molecular pattern
- ECM:
-
Extracellular matrix
- GAG:
-
Glycosaminoglycan
- HMGB1:
-
High mobility group box 1
- SIS:
-
Small intestinal submucosa
- TE/RM:
-
Tissue Engineering and Regenerative Medicine
- TLR:
-
Toll-like receptor
- TNFα:
-
Tumor necrosis factor alpha
References
Agrawal, V., S. A. Johnson, J. Reing, L. Zhang, S. Tottey, et al. Epimorphic regeneration approach to tissue replacement in adult mammals. Proc Natl Acad Sci U S A 107:3351–3355, 2010.
Alvarado, A. Regeneration in the metazoans: why does it happen? BioEssays 22:578–590, 2000.
Anderson, J. M. Inflammatory response to implants. ASAIO Trans 34:101–107, 1988.
Anderson, J. M., A. Rodriguez, and D. T. Chang. Foreign body reaction to biomaterials. Semin Immunol 20:86–100, 2008.
Andrassy, M., H. C. Volz, J. C. Igwe, B. Funke, S. N. Eichberger, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 117:3216–3226, 2008.
Badylak, S. F., T. Hoppo, A. Nieponice, T. W. Gilbert, J. M. Davison, and B. A. Jobe. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A 17:1643–1650, 2011.
Badylak, S., K. Kokini, B. Tullius, A. Simmons-Byrd, and R. Morff. Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res 103:190–202, 2002.
Badylak, S., K. Kokini, B. Tullius, and B. Whitson. Strength over time of a resorbable bioscaffold for body wall repair in a dog model. J Surg Res 99:282–287, 2001.
Badylak, S. F., G. C. Lantz, A. Coffey, and L. A. Geddes. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res 47:74–80, 1989.
Badylak, S. F., K. Park, N. Peppas, G. McCabe, and M. Yoder. Marrow-derived cells populate scaffolds composed of xenogeneic extracellular matrix. Exp Hematol 29:1310–1318, 2001.
Beattie, A. J., T. W. Gilbert, J. P. Guyot, A. J. Yates, and S. F. Badylak. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng Part A 15:1119–1125, 2009.
Brown, B. N., and S. F. Badylak. Expanded applications, shifting paradigms and an improved understanding of host-biomaterial interactions. Acta Biomater 9:4948–4955, 2013.
Brown, B. N., J. M. Freund, L. Han, J. P. Rubin, J. E. Reing, et al. Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Eng Part C Methods 17:411–421, 2011.
Brown, B. N., R. Londono, S. Tottey, L. Zhang, K. A. Kukla, et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater 8:978–987, 2012.
Brown, B. N., B. D. Ratner, S. B. Goodman, S. Amar, and S. F. Badylak. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 33:3792–3802, 2012.
Brown, B. N., J. E. Valentin, A. M. Stewart-Akers, G. P. McCabe, and S. F. Badylak. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 30:1482–1491, 2009.
Cartmell, J. S., and M. G. Dunn. Effect of chemical treatments on tendon cellularity and mechanical properties. J Biomed Mater Res 49:134–140, 2000.
Choi, J. S., J. K. Williams, M. Greven, K. A. Walter, P. W. Laber, et al. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials 31:6738–6745, 2010.
Clough, A., J. Ball, G. S. Smith, and S. Leibman. Porcine small intestine submucosa matrix (Surgisis) for esophageal perforation. Ann Thorac Surg 91:e15–e16, 2011.
Cobb, M. A., S. F. Badylak, W. Janas, and F. A. Boop. 1996. Histology after dural grafting with small intestinal submucosa. Surg. Neurol. 46:389–393; discussion 93-4.
Colorado, P. C., A. Torre, G. Kamphaus, Y. Maeshima, H. Hopfer, et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res 60:2520–2526, 2000.
Crapo, P. M., T. W. Gilbert, and S. F. Badylak. An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243, 2011.
Daly, K., A. Stewart-Akers, H. Hara, M. Ezzelarab, C. Long, et al. Effect of the alphaGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue Eng. Part A. 15(12), 3877–3888, 2009.
Daly, K. A., S. Liu, V. Agrawal, B. N. Brown, S. A. Johnson, et al. Damage associated molecular patterns within xenogeneic biologic scaffolds and their effects on host remodeling. Biomaterials 33:91–101, 2012.
de Castro Bras, L. E., S. Shurey, and P. D. Sibbons. Evaluation of crosslinked and non-crosslinked biologic prostheses for abdominal hernia repair. Hernia 16:77–89, 2012.
De Mori, R., S. Straino, A. Di Carlo, A. Mangoni, G. Pompilio, et al. Multiple effects of high mobility group box protein 1 in skeletal muscle regeneration. Arterioscler Thromb Vasc Biol 27:2377–2383, 2007.
Deeken, C. R., A. K. White, S. L. Bachman, B. J. Ramshaw, D. S. Cleveland, et al. 2010. Method of preparing a decellularized porcine tendon using tributyl phosphate. J. Biomed. Mater. Res. B Appl. Biomater.
Dennis, C. Brief history of development of vascular grafts. In: Modern Vascular Grafts, edited by P. Sawyer. New York: McGraw-Hill, 1987, 326 pp.
Dong, X., X. Wei, W. Yi, C. Gu, X. Kang, et al. 2009. RGD-modified acellular bovine pericardium as a bioprosthetic scaffold for tissue engineering. J. Mater. Sci. Mater. Med.
Fitzpatrick, J. C., P. M. Clark, F. M. Capaldi. 2010. Effect of decellularization protocol on the mechanical behavior of porcine descending aorta. Int. J. Biomater. 2010.
Flynn, L. E. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 31:4715–4724, 2010.
Gilbert, T. W., M. S. Sacks, J. S. Grashow, S. L. Woo, S. F. Badylak, and M. B. Chancellor. Fiber kinematics of small intestinal submucosa under biaxial and uniaxial stretch. J Biomech Eng 128:890–898, 2006.
Gilbert, T. W., T. L. Sellaro, and S. F. Badylak. Decellularization of tissues and organs. Biomaterials 27:3675–3683, 2006.
Gillies, A. R., L. R. Smith, R. L. Lieber, and S. Varghese. Method for decellularizing skeletal muscle without detergents or proteolytic enzymes. Tissue Eng Part C Methods 17:383–389, 2011.
Gordon, S., and P. R. Taylor. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964, 2005.
Gratzer, P. F., R. D. Harrison, and T. Woods. Matrix alteration and not residual sodium dodecyl sulfate cytotoxicity affects the cellular repopulation of a decellularized matrix. Tissue Eng 12:2975–2983, 2006.
Grauss, R. W., M. G. Hazekamp, F. Oppenhuizen, C. J. van Munsteren, A. C. Gittenberger-de Groot, and M. C. DeRuiter. 2005. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur. J. Cardiothorac. Surg. 27:566–571.
Ho, K. L., M. N. Witte, and E. T. Bird. 8-ply small intestinal submucosa tension-free sling: spectrum of postoperative inflammation. J Urol 171:268–271, 2004.
Hodde, J. P., S. F. Badylak, and K. D. Shelbourne. The effect of range of motion on remodeling of small intestinal submucosa (SIS) when used as an Achilles tendon repair material in the rabbit. Tissue Eng 3:27–37, 1997.
Horowitz, B., R. Bonomo, A. M. Prince, S. N. Chin, B. Brotman, and R. W. Shulman. Solvent/detergent-treated plasma: a virus-inactivated substitute for fresh frozen plasma. Blood 79:826–831, 1992.
Huang, M., N. Li, Z. Wu, P. Wan, X. Liang, et al. Using acellular porcine limbal stroma for rabbit limbal stem cell microenvironment reconstruction. Biomaterials 32:7812–7821, 2011.
Hudson, T. W., S. Y. Liu, and C. E. Schmidt. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng 10:1346–1358, 2004.
Keane, T. J., R. Londono, N. J. Turner, and S. F. Badylak. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 33:1771–1781, 2012.
Lantz, G. C., S. F. Badylak, A. C. Coffey, L. A. Geddes, and W. E. Blevins. Small intestinal submucosa as a small-diameter arterial graft in the dog. J Invest Surg 3:217–227, 1990.
Lawrence, T., and G. Natoli. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11:750–761, 2011.
Levy, R. J., N. Vyavahare, M. Ogle, P. Ashworth, R. Bianco, and F. J. Schoen. 2003. Inhibition of cusp and aortic wall calcification in ethanol- and aluminum-treated bioprosthetic heart valves in sheep: background, mechanisms, and synergism. J. Heart Valve Dis. 12:209–216; discussion 16.
Liao, J., L. Yang, J. Grashow, and M. S. Sacks. The relation between collagen fibril kinematics and mechanical properties in the mitral valve anterior leaflet. J Biomech Eng 129:78–87, 2007.
Limana, F., A. Germani, A. Zacheo, J. Kajstura, A. Di Carlo, et al. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res 97:e73–e83, 2005.
Lolmede, K., L. Campana, M. Vezzoli, L. Bosurgi, R. Tonlorenzi, et al. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol 85:779–787, 2009.
Longaker, M. T., M. R. Harrison, T. M. Crombleholme, J. C. Langer, M. Decker, et al. Studies in fetal wound healing: I. A factor in fetal serum that stimulates deposition of hyaluronic acid. J Pediatr Surg 24:789–792, 1989.
Longaker, M. T., D. J. Whitby, M. W. Ferguson, M. R. Harrison, T. M. Crombleholme, et al. Studies in fetal wound healing: III. Early deposition of fibronectin distinguishes fetal from adult wound healing. J Pediatr Surg 24:799–805, 1989.
Lumpkins, S. B., N. Pierre, and P. S. McFetridge. A mechanical evaluation of three decellularization methods in the design of a xenogeneic scaffold for tissue engineering the temporomandibular joint disc. Acta Biomater 4:808–816, 2008.
Lynch, A. P., and M. Ahearne. Strategies for developing decellularized corneal scaffolds. Exp Eye Res 108:42–47, 2013.
Mantovani, A., A. Sica, S. Sozzani, P. Allavena, A. Vecchi, and M. Locati. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686, 2004.
Martin, P., and J. Lewis. Actin cables and epidermal movement in embryonic wound healing. Nature 360:179–183, 1992.
McPherson, T. B., H. Liang, R. D. Record, and S. F. Badylak. Galalpha(1,3)Gal epitope in porcine small intestinal submucosa. Tissue Eng 6:233–239, 2000.
Meyer, S. R., B. Chiu, T. A. Churchill, L. Zhu, J. R. Lakey, and D. B. Ross. Comparison of aortic valve allograft decellularization techniques in the rat. J Biomed Mater Res A 79:254–262, 2006.
Mills, C. D., K. Kincaid, J. M. Alt, M. J. Heilman, and A. M. Hill. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164:6166–6173, 2000.
Montoya, C. V., and P. S. McFetridge. Preparation of ex vivo-based biomaterials using convective flow decellularization. Tissue Eng Part C Methods 15:191–200, 2009.
Morgan, T. Regeneration in the Egg, Embryo, and Adult. The American Naturalist 35:949–979, 1901.
Mosser, D. M., and J. P. Edwards. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969, 2008.
Musahl, V., S. D. Abramowitch, T. W. Gilbert, E. Tsuda, J. H. Wang, et al. The use of porcine small intestinal submucosa to enhance the healing of the medial collateral ligament—a functional tissue engineering study in rabbits. J Orthop Res 22:214–220, 2004.
Nakayama, K. H., C. A. Batchelder, C. I. Lee, and A. F. Tarantal. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A 16:2207–2216, 2010.
O’Neill, J. D., R. Anfang, A. Anandappa, J. Costa, J. Javidfar, et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg 96:1046–1055; discussion 55–56. 2013.
O’Reilly, M. S., T. Boehm, Y. Shing, N. Fukai, G. Vasios, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88:277–285, 1997.
Ozeki, M., Y. Narita, H. Kagami, N. Ohmiya, A. Itoh, et al. Evaluation of decellularized esophagus as a scaffold for cultured esophageal epithelial cells. J Biomed Mater Res A 79:771–778, 2006.
Petersen, T. H., E. A. Calle, M. B. Colehour, and L. E. Niklason. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs 195:222–231, 2012.
Petersen, T. H., E. A. Calle, L. Zhao, E. J. Lee, L. Gui, et al. Tissue-engineered lungs for in vivo implantation. Science 329:538–541, 2010.
Petter-Puchner, A. H., R. H. Fortelny, R. Mittermayr, N. Walder, W. Ohlinger, and H. Redl. Adverse effects of porcine small intestine submucosa implants in experimental ventral hernia repair. Surg Endosc 20:942–946, 2006.
Ponce Marquez, S., V. S. Martinez, W. McIntosh Ambrose, J. Wang, N. G. Gantxegui, et al. Decellularization of bovine corneas for tissue engineering applications. Acta Biomater 5:1839–1847, 2009.
Porto, A., R. Palumbo, M. Pieroni, G. Aprigliano, R. Chiesa, et al. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J 20:2565–2566, 2006.
Prasertsung, I., S. Kanokpanont, T. Bunaprasert, V. Thanakit, and S. Damrongsakkul. Development of acellular dermis from porcine skin using periodic pressurized technique. J Biomed Mater Res B Appl Biomater 85:210–219, 2008.
Ramchandran, R., M. Dhanabal, R. Volk, M. J. Waterman, M. Segal, et al. Antiangiogenic activity of restin, NC10 domain of human collagen XV: comparison to endostatin. Biochem Biophys Res Commun 255:735–739, 1999.
Ranzato, E., M. Patrone, M. Pedrazzi, and B. Burlando. HMGb1 promotes scratch wound closure of HaCaT keratinocytes via ERK1/2 activation. Mol Cell Biochem 332:199–205, 2009.
Record, R. D., D. Hillegonds, C. Simmons, R. Tullius, F. A. Rickey, et al. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials 22:2653–2659, 2001.
Reing, J. E., B. N. Brown, D. A. Daly, J. M. Freund, T. W. Gilbert, et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 31:8626–8633, 2010.
Reing, J. E., L. Zhang, J. Myers-Irvin, K. E. Cordero, D. O. Freytes, et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A 15:605–614, 2009.
Rieder, E., M. T. Kasimir, G. Silberhumer, G. Seebacher, E. Wolner, et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg 127:399–405, 2004.
Sarikaya, A., R. Record, C. C. Wu, B. Tullius, S. Badylak, and M. Ladisch. Antimicrobial activity associated with extracellular matrices. Tissue Eng 8:63–71, 2002.
Sheridan, W. S., G. P. Duffy, and B. P. Murphy. Mechanical characterization of a customized decellularized scaffold for vascular tissue engineering. J Mech Behav Biomed Mater 8:58–70, 2012.
Sicari, B. M., S. A. Johnson, B. F. Siu, P. M. Crapo, K. A. Daly, et al. The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials 33:5524–5533, 2012.
Stout, R. D., S. K. Watkins, and J. Suttles. Functional plasticity of macrophages: in situ reprogramming of tumor-associated macrophages. J Leukoc Biol 86:1105–1109, 2009.
Thomas, J. O. HMG1 and 2: architectural DNA-binding proteins. Biochem Soc Trans 29:395–401, 2001.
Tian, J., A. M. Avalos, S. Y. Mao, B. Chen, K. Senthil, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 8:487–496, 2007.
Tottey, S., S. A. Johnson, P. M. Crapo, J. E. Reing, L. Zhang, et al. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials 32:128–136, 2011.
Turner, N. J., A. J. Yates, Jr, D. J. Weber, I. R. Qureshi, D. B. Stolz, et al. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng Part A 16:3309–3317, 2010.
Uygun, B. E., A. Soto-Gutierrez, H. Yagi, M. L. Izamis, M. A. Guzzardi, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 16:814–820, 2010.
Valentin, J. E., A. M. Stewart-Akers, T. W. Gilbert, and S. F. Badylak. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng Part A 15:1687–1694, 2009.
Valentin, J. E., N. J. Turner, T. W. Gilbert, and S. F. Badylak. Functional skeletal muscle formation with a biologic scaffold. Biomaterials 31:7475–7484, 2010.
Vavken, P., S. Joshi, and M. M. Murray. TRITON-X is most effective among three decellularization agents for ACL tissue engineering. J Orthop Res 27:1612–1618, 2009.
Whitby, D. J., and M. W. Ferguson. The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development 112:651–668, 1991.
Woods, T., and P. F. Gratzer. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials 26:7339–7349, 2005.
Xu, C. C., R. W. Chan, and N. Tirunagari. A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria. Tissue Eng. 2006.
Yang, D., Q. Chen, H. Yang, K. J. Tracey, M. Bustin, and J. J. Oppenheim. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol 81:59–66, 2007.
Zhang, A. Y., S. J. Bates, E. Morrow, H. Pham, B. Pham, and J. Chang. Tissue-engineered intrasynovial tendons: optimization of acellularization and seeding. J Rehabil Res Dev 46:489–498, 2009.
Zhang, Q., M. Raoof, Y. Chen, Y. Sumi, T. Sursal, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464:104–107, 2010.
Zhou, J., O. Fritze, M. Schleicher, H. P. Wendel, K. Schenke-Layland, et al. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials 31:2549–2554, 2010.
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Song Li oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Badylak, S.F. Decellularized Allogeneic and Xenogeneic Tissue as a Bioscaffold for Regenerative Medicine: Factors that Influence the Host Response. Ann Biomed Eng 42, 1517–1527 (2014). https://doi.org/10.1007/s10439-013-0963-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-013-0963-7