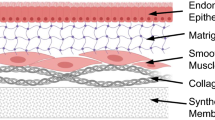
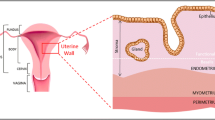
Abstract
Research insights into uterine function and the mechanisms of labour have been hindered by the lack of suitable animal and cellular models. The use of traditional culturing methods limits the exploration of complex uterine functions, such as cell interactions, connectivity and contractile behaviour, as it fails to mimic the three-dimensional (3D) nature of uterine cell interactions in vivo. Animal models are an option, however, use of these models is constrained by ethical considerations as well as translational limitations to humans. Evidence indicates that these limitations can be overcome by using 3D culture systems, or 3D Bioprinters, to model the in vivo cytological architecture of the tissue in an in vitro environment. 3D cultured or 3D printed cells can be used to form an artificial tissue. This artificial tissue can not only be used as an appropriate model in which to study cellular function and organisation, but could also be used for regenerative medicine purposes including organ or tissue transplantation, organ donation and obstetric care. The current review describes recent developments in cell culture that can facilitate the development of myometrial 3D structures and tissue engineering applications.
Similar content being viewed by others
References
Adissu, H. A., E. K. Asem, and S. A. Lelievre. Three-dimensional cell culture to model epithelia in the female reproductive system. Reprod. Sci. 14:11–19, 2007.
Almany, L., and D. Seliktar. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 26:2467–2477, 2005.
Astashkina, A., B. Mann, and D. W. Grainger. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol. Ther. 134:82–106, 2012.
Bajpai, V. K., P. Mistriotis, Y. H. Loh, G. Q. Daley, and S. T. Andreadis. Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc. Res. 96:391–400, 2012.
Boretti, M. I., and K. J. Gooch. Effect of extracellular matrix and 3D morphogenesis on islet hormone gene expression by Ngn3-infected mouse pancreatic ductal epithelial cells. Tissue Eng. A 14:1927–1937, 2008.
Bursztyn, L., O. Eytan, A. J. Jaffa, and D. Elad. Mathematical model of excitation-contraction in a uterine smooth muscle cell. Am. J. Physiol. Cell Physiol. 292:C1816–C1829, 2007.
Castillejo, M., E. Rebollar, M. Oujja, M. Sanz, A. Selimis, M. Sigletou, S. Psycharakis, A. Ranella, and C. Fotakis. Fabrication of porous biopolymer substrates for cell growth by UV laser: The role of pulse duration. Appl. Surf. Sci. 258:8919–8927, 2012.
Chamley-Campbell, J., G. R. Campbell, and R. Ross. The smooth muscle cell in culture. Physiol. Rev. 59:1–61, 1979.
Charwat, V., K. Schütze, W. Holnthoner, A. Lavrentieva, R. Gangnus, P. Hofbauer, C. Hoffmann, B. Angres, and C. Kasper. Potential and limitations of microscopy and Raman spectroscopy for live-cell analysis of 3D cell cultures. J. Biotechnol. 205:70–81, 2015.
Condon, J., S. Yin, B. Mayhew, R. A. Word, W. E. Wright, J. W. Shay, and W. E. Rainey. Telomerase immortalization of human myometrial cells. Biol. Reprod. 67(2):506–514, 2002.
Dainiak, M. B., I. N. Savina, I. Musolino, A. Kumar, B. Mattiasson, and I. Y. Galaev. Biomimetic macroporous hydrogel scaffolds in a high-throughput screening format for cell-based assays. Biotechnol. Prog. 24:1373–1383, 2008.
Dallot, E., M. Pouchelet, N. Gouhier, D. Cabrol, F. Ferre, and M. Breuiller-Fouche. Contraction of cultured human uterine smooth muscle cells after stimulation with endothelin-1. Biol. Reprod. 68:937–942, 2003.
Dhiman, H. K., A. R. Ray, and A. K. Panda. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials 26:979–986, 2005.
Drover, J. W., and R. F. Casper. Initiation of parturition in humans. Can. Med. Assoc. J. 128:387–392, 1983.
Elliott, N. T., and F. Yuan. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J. Pharm. Sci. 100:59–74, 2011.
El-Sherbiny, I. M., and M. H. Yacoub. Hydrogel scaffolds for tissue engineering: Progress and challenges. Global Cardiol. Sci. Pract. 2013:38, 2013.
Equils, O., P. Nambiar, C. J. Hobel, R. Smith, C. F. Simmons, and S. Vali. A computer simulation of progesterone and Cox2 inhibitor treatment for preterm labor. PLoS ONE 5:e8502, 2010.
Fitzgibbon, J., J. J. Morrison, T. J. Smith, and M. O’Brien. Modulation of human uterine smooth muscle cell collagen contractility by thrombin, Y-27632, TNF alpha and indomethacin. Reprod. Biol. Endocrinol. 7:2, 2009.
Fleischer, S., J. Miller, H. Hurowitz, A. Shapira, and T. Dvir. Effect of fiber diameter on the assembly of functional 3D cardiac patches. Nanotechnology 26(29):291002, 2015.
Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J. Vis. Exp. 51:2720, 2011.
Gong, P. Y., W. Zheng, D. Xiao, and X. Y. Jiang. Microscale methods to assemble mammalian cells into tissue-like structures. Sci. China Life Sci. 55:862–871, 2012.
Graf, B. W., and S. A. Boppart. Imaging and analysis of three-dimensional cell culture models. Methods Mol. Biol. 591:211–227, 2010.
Grefte, S., S. Vullinghs, A. M. Kuijpers-Jagtman, R. Torensma, and J. W. Von den Hoff. Matrigel, but not collagen I, maintains the differentiation capacity of muscle derived cells in vitro. Biomed. Mater. 7:055004, 2012.
Grima, R. Directed cell migration in the presence of obstacles. Theor. Biol. Med. Model. 4:2, 2007.
Harris, L. J., H. Abdollahi, P. Zhang, S. McIlhenny, T. N. Tulenko, and P. J. DiMuzio. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J. Surg. Res. 168:306–314, 2011.
Hellström, M., R. R. El-Akouri, C. Sihlbom, B. M. Olsson, J. Lengqvist, H. Bäckdahl, B. R. Johansson, M. Olausson, S. Sumitran-Holgersson, and M. Brännström. Towards the development of a bioengineered uterus: Comparison of different protocols for rat uterus decellularization. Acta Biomater. 10:5034–5042, 2014.
Hellström, M., J. M. Moreno-Moya, S. Bandstein, E. Bom, R. R. Akouri, K. Miyazaki, T. Maruyama, and M. Brännström. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil. Steril. 106(2):487–496, 2016.
Holmes, T. C., S. de Lacalle, X. Su, G. Liu, A. Rich, and S. Zhang. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc. Natl Acad. Sci. USA 97:6728–6733, 2000.
Hsieh, W.-C., and J.-J. Liau. Cell culture and characterization of cross-linked poly(vinyl alcohol)-g-starch 3D scaffold for tissue engineering. Carbohydr. Polym. 98:574–580, 2013.
Huh, D., G. A. Hamilton, and D. E. Ingber. From 3D cell culture to organs-on-chips. Trends Cell Biol. 21:745–754, 2011.
Ikonen, L., E. Kerkelä, G. Metselaar, M. C. A. Stuart, M. R. de Jong, and K. Aalto-Setälä. 2D and 3D self-assembling nanofiber hydrogels for cardiomyocyte culture. BioMed. Res. Int. 2013. doi:10.1155/2013/285678.
Kakade, S., and G. Mani. A comparative study of the effects of vitamin C, sirolimus, and paclitaxel on the growth of endothelial and smooth muscle cells for cardiovascular medical device applications. Drug Des. Dev. Ther. 7:529–544, 2013.
Heidari Kani, M., R. Smith, T. Butler, C. Chan, and R. Young. Glass wool as a model scaffold for 3D culture of uterine smooth muscle cells. Front. Bioeng. Biotechnol. Conference Abstract: 10th World Biomaterials Congress. 2016. doi:10.3389/conf.FBIOE.2016.01.02608.
Khait, L., C. J. Hodonsky, and R. K. Birla. Variable optimization for the formation of three-dimensional self-organized heart muscle. Vitro Cell. Dev. Biol. 45:592–601, 2009.
Khetan, S., and J. Burdick. Cellular encapsulation in 3D hydrogels for tissue engineering. J. Vis. Exp. 2009. doi:10.3791/1590.
Khoshfetrat, Pakazad S. A. Savov, A. Van De Stolpe and R. Dekker. A novel stretchable micro-electrode array (SMEA) design for directional stretching of cells. J. Micromech. Microeng. 24(3):034003, 2014.
Kucukgul, C., B. Ozler, H. E. Karakas, D. Gozuacik, and B. Koc. 3D hybrid bioprinting of macrovascular structures. Procedia Eng. 59:183–192, 2013.
Kuo, C. W., D. Y. Chueh, and P. Chen. Investigation of size-dependent cell adhesion on nanostructured interfaces. J. Nanobiotechnol. 12:54, 2014.
Lawrence, B. J., and S. V. Madihally. Cell colonization in degradable 3D porous matrices. Cell Adhens. Migr. 2:9–16, 2008.
Lee, H. J., E. R. Norwitz, and J. Shaw. Contemporary management of fibroids in pregnancy. Rev. Obstet. Gynecol. 3:20–27, 2010.
Lee, V., G. Singh, J. P. Trasatti, C. Bjornsson, X. Xu, T. N. Tran, S. S. Yoo, G. Dai, and P. Karande. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. C Methods 20:473–484, 2014.
Lee, J. B., S. H. Son, M. C. Park, T. H. Kim, M. G. Kim, S. D. Yoo, and S. Kim. A novel in vitro permeability assay using three-dimensional cell culture system. J. Biotechnol. 205:93–100, 2015.
Lee, D. W., S. H. Yi, S. H. Jeong, B. Ku, J. Kim, and M.-Y. Lee. Plastic pillar inserts for three-dimensional (3D) cell cultures in 96-well plates. Sens. Actuators B 177:78–85, 2013.
Lei, K. F., M. H. Wu, C. W. Hsu, and Y. D. Chen. Real-time and non-invasive impedimetric monitoring of cell proliferation and chemosensitivity in a perfusion 3D cell culture microfluidic chip. Biosens. Bioelectron. 51:16–21, 2013.
Li, Z., and Z. Cui. Three-dimensional perfused cell culture. Biotechnol. Adv. 32:243–254, 2014.
Liu, J. Y., H. F. Peng, and S. T. Andreadis. Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc. Res. 79:24–33, 2008.
Lovitt, C. J., T. B. Shelper, and V. M. Avery. Advanced cell culture techniques for cancer drug discovery. Biology 3:345–367, 2014.
Masuda, S., and T. Shimizu. Three-dimensional cardiac tissue fabrication based on cell sheet technology. Adv. Drug Deliv. Rev. 96:103–109, 2016.
Mironov, V., T. Boland, T. Trusk, G. Forgacs, and R. R. Markwald. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 21:157–161, 2003.
Mironov, V., V. Kasyanov, and R. R. Markwald. Organ printing: from bioprinter to organ biofabrication line. Curr. Opin. Biotechnol. 22:667–673, 2011.
Mironov, V., N. Reis, and B. Derby. Review: bioprinting: a beginning. Tissue Eng. 12:631–634, 2006.
Mitchell, B. F., and M. J. Taggart. Are animal models relevant to key aspects of human parturition? Am. J. Physiol. 297:R525–R545, 2009.
Miyazaki, K., and T. Maruyama. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials 35:8791–8800, 2014.
Monga, M., C. Y. Ku, K. Dodge, and B. M. Sanborn. Oxytocin-stimulated responses in a pregnant human immortalized myometrial cell line. Biol. Reprod. 55:427–432, 1996.
Negishi, J., S. Funamoto, T. Kimura, K. Nam, T. Higami, and A. Kishida. Effect of treatment temperature on collagen structures of the decellularized carotid artery using high hydrostatic pressure. J. Artif. Organs 14:223–231, 2011.
Norotte, C., F. S. Marga, L. E. Niklason, and G. Forgacs. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30:5910–5917, 2009.
Ong, S.-M., C. Zhang, Y.-C. Toh, S. H. Kim, H. L. Foo, C. H. Tan, D. van Noort, S. Park, and H. Yu. A gel-free 3D microfluidic cell culture system. Biomaterials 29:3237–3244, 2008.
Palumbo, F. S., G. Pitarresi, C. Fiorica, S. Rigogliuso, G. Ghersi, and G. Giammona. Chemical hydrogels based on a hyaluronic acid-graft-α-elastin derivative as potential scaffolds for tissue engineering. Mater. Sci. Eng. C 33:2541–2549, 2013.
Park, W. S., S. C. Heo, E. S. Jeon, D. H. Hong, Y. K. Son, J. H. Ko, H. K. Kim, S. Y. Lee, J. H. Kim, and J. Han. Functional expression of smooth muscle-specific ion channels in TGF-β1-treated human adipose-derived mesenchymal stem cells. Am. J. Physiol. 305:C377–C391, 2013.
Pati, F., J.-H. Shim, J.-S. Lee, and D.-W. Cho. 3D printing of cell-laden constructs for heterogeneous tissue regeneration. Manuf. Lett. 1:49–53, 2013.
Rampichová, M., J. Chvojka, M. Buzgo, E. Prosecká, P. Mikeš, L. Vysloužilová, D. Tvrdík, P. Kochová, T. Gregor, D. Lukáš, and E. Amler. Elastic three-dimensional poly (ε-caprolactone) nanofibre scaffold enhances migration, proliferation and osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 46:23–37, 2013.
Ravi, M., V. Paramesh, S. R. Kaviya, E. Anuradha, and F. D. Paul Solomon. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 230:16–26, 2015.
Schindler, M., A. Nur-E-Kamal, I. Ahmed, J. Kamal, H. Y. Liu, N. Amor, A. S. Ponery, D. P. Crockett, T. H. Grafe, H. Y. Chung, T. Weik, E. Jones, and S. Meiners. Living in three dimensions: 3D nanostructured environments for cell culture and regenerative medicine. Cell Biochem. Biophys. 45:215–227, 2006.
Shamir, E. R., and A. J. Ewald. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol. 15:647–664, 2014.
Shay, J. W., and W. E. Wright. Use of telomerase to create bioengineered tissues. Ann. N. Y. Acad. Sci. 479–491:2005, 1057.
Shrestha, K. R., Y. H. Park, Y. S. Choi, I. G. Kim, S. Piao, A. R. Jung, S. H. Jeon, S. H. Oh, J. H. Lee, and J. Y. Lee. Bladder reconstruction using stem cells seeded on multilayered scaffolds in a mucosa preserving partial cystectomy model. Tissue Eng. Regener. Med. 12:427–434, 2015.
Shynlova, O., P. Tsui, S. Jaffer, and S. J. Lye. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur. J. Obstet. Gynecol. Reprod. Biol. 144(Supplement 1):S2–S10, 2009.
Simon, K. A., K. M. Park, B. Mosadegh, A. B. Subramaniam, A. D. Mazzeo, P. M. Ngo, and G. M. Whitesides. Polymer-based mesh as supports for multi-layered 3D cell culture and assays. Biomaterials 35:259–268, 2014.
Sims, S. M., E. E. Daniel, and R. E. Garfield. Improved electrical coupling in uterine smooth muscle is associated with increased numbers of gap junctions at parturition. J. Gen. Physiol. 80:353–375, 1982.
Sokolowski, P., F. Saison, W. Giles, S. McGrath, D. Smith, J. Smith, and R. Smith. Human uterine wall tension trajectories and the onset of parturition. PLoS ONE 5:e11037, 2010.
Stile, R. A., W. R. Burghardt, and K. E. Healy. Synthesis and characterization of injectable poly(N-isopropylacrylamide)-based hydrogels that support tissue formation in vitro. Macromolecules 32:7370–7379, 1999.
Stratmann, A. T., D. Fecher, G. Wangorsch, C. Göttlich, T. Walles, H. Walles, T. Dandekar, G. Dandekar, and S. L. Nietzer. Establishment of a human 3D lung cancer model based on a biological tissue matrix combined with a Boolean in silico model. Mol. Oncol. 8:351–365, 2014.
Sullivan, D. C., S. H. Mirmalek-Sani, D. B. Deegan, P. M. Baptista, T. Aboushwareb, A. Atala, and J. J. Yoo. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials 33:7756–7764, 2012.
Sylvain, C., F. Jean-Christophe, G. Bertrand, P. Benjamin, B. Reine, R. Murielle, L. Eric, D. Bernard, A. Joëlle, and G. Fabien. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication 3:025001, 2011.
Taggart, M. J., A. Blanks, S. Kharche, A. Holden, B. Wang, and H. Zhang. Towards understanding the myometrial physiome: approaches for the construction of a virtual physiological uterus. BMC Pregnancy Childbirth 7(Suppl 1):S3, 2007.
Tamayol, A., M. Akbari, N. Annabi, A. Paul, A. Khademhosseini, and D. Juncker. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnol. Adv. 31:669–687, 2013.
Tan, Y., D. J. Richards, T. C. Trusk, R. P. Visconti, M. J. Yost, M. S. Kindy, C. J. Drake, W. S. Argraves, R. R. Markwald, and Y. Mei. 3D printing facilitated scaffold-free tissue unit fabrication. Biofabrication 6:024111, 2014.
Topman, G., N. Shoham, O. Sharabani-Yosef, F. H. Lin, and A. Gefen. A new technique for studying directional cell migration in a hydrogel-based three-dimensional matrix for tissue engineering model systems. Micron 51:9–12, 2013.
Turnbull, A. C. Myometrial contractility in pregnancy and its regulation. Proc R. Soc. Med. 64:1015–1017, 1971.
Turner, W. S., N. Sandhu, and K. E. McCloskey. Tissue engineering: Construction of a multicellular 3D scaffold for the delivery of layered cell sheets. J. Visualized Exp. 92:e51044–e51044, 2014.
Wang, Z., Y. Cui, J. Wang, X. Yang, Y. Wu, K. Wang, X. Gao, D. Li, Y. Li, X. L. Zheng, Y. Zhu, D. Kong, and Q. Zhao. The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 35:5700–5710, 2014.
Wang, L., L. Liu, X. Li, N. Magome, K. Agladze, and Y. Chen. Multi-electrode monitoring of guided excitation in patterned cardiomyocytes. Microelectron. Eng. 111:267–271, 2013.
Wozniak, M. A., and P. J. Keely. Use of three-dimensional collagen gels to study mechanotransduction in T47D breast epithelial cells. Biol. Proced. Online 7:144–161, 2005.
Xiao, Q., G. Wang, Z. Luo, and Q. Xu. The mechanism of stem cell differentiation into smooth muscle cells. Thromb. Haemost. 104:440–448, 2010.
Xu, T., H. Kincaid, A. Atala, and J. J. Yoo. High-throughput production of single-cell microparticles using an inkjet printing technology. Trans. ASME Ser. B 130:210171–210175, 2008.
Xu, T., J. I. Rodriguez-Devora, D. Reyna-Soriano, M. Bhuyan, L. Zhu, K. Wang, and Y. Yuan. Chapter 6 - Principles of Bioprinting Technology. In: Regenerative medicine applications in organ transplantation, edited by G. Orlando, J. Lerut, S. Soker, and R. J. Stratta. Boston: Academic Press, 2014, pp. 67–79.
Yamamoto, M., N. Kawashima, N. Takashino, Y. Koizumi, K. Takimoto, N. Suzuki, M. Saito, and H. Suda. Three-dimensional spheroid culture promotes odonto/osteoblastic differentiation of dental pulp cells. Arch. Oral Biol. 59:310–317, 2014.
Yan, P., C. Xia, C. Duan, S. Li, and Z. Mei. Biological characteristics of foam cell formation in smooth muscle cells derived from Bone Marrow stem cells. Int. J. Biol. Sci. 7:937–946, 2011.
Yoshii, Y., A. Waki, K. Yoshida, A. Kakezuka, M. Kobayashi, H. Namiki, Y. Kuroda, Y. Kiyono, H. Yoshii, T. Furukawa, T. Asai, H. Okazawa, J. G. Gelovani, and Y. Fujibayashi. The use of nanoimprinted scaffolds as 3D culture models to facilitate spontaneous tumor cell migration and well-regulated spheroid formation. Biomaterials 32:6052–6058, 2011.
Young, R. C. Myocytes, myometrium, and uterine contractions. Ann. N. Y. Acad. Sci. 1101:72–84, 2007.
Young, R. C., R. Schumann, and P. Zhang. Three-dimensional culture of human uterine smooth muscle myocytes on a resorbable scaffolding. Tissue Eng. 9:451–459, 2003.
Yu, C., Z. Zhu, L. Wang, Q. Wang, N. Bao, and H. Gu. A new disposable electrode for electrochemical study of leukemia K562 cells and anticancer drug sensitivity test. Biosens. Bioelectron. 53:142–147, 2014.
Zhang, S., F. Gelain, and X. Zhao. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin. Cancer Biol. 15:413–420, 2005.
Zhao, X., L. Liu, J. Wang, Y. Xu, W. Zhang, G. Khang, and X. Wang. In vitro vascularization of a combined system based on a 3D printing technique. J Tissue Eng Regen Med. 2014. doi:10.1002/term.1863.
Zhao, Z. K., H. L. Yu, F. Xiao, S. W. Li, W. B. Liao, and K. L. Zhao. Muscle-derived stem cells differentiate into functional smooth muscle cells for ureter tissue engineering: An experimental study. Biotechnol. Bioprocess Eng. 17:456–464, 2012.
Acknowledgements
This work was conducted with the support of an Australian NHMRC grant to Roger Smith (G1200367).
Conflict of Interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Christiani Amorim oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Heidari Kani, M., Chan, EC., Young, R.C. et al. 3D Cell Culturing and Possibilities for Myometrial Tissue Engineering. Ann Biomed Eng 45, 1746–1757 (2017). https://doi.org/10.1007/s10439-016-1749-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1749-5