Abstract
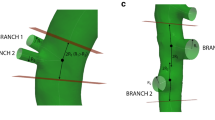
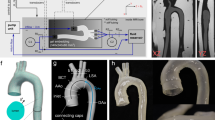
In order to advance the state-of-the-art in computational aortic biomechanics, we investigated the influence of (i) a non-uniform wall thickness, (ii) minor aortic side branches and (iii) a non-uniform axial stretch distribution on the location of predicted hotspots of principal strain in a mouse model for dissecting aneurysms. After 3 days of angiotensin II infusion, a murine abdominal aorta was scanned in vivo with contrast-enhanced micro-CT. The animal was subsequently sacrificed and its aorta was scanned ex vivo with phase-contrast X-ray tomographic microscopy (PCXTM). An automatic morphing framework was developed to map the non-pressurized, non-stretched PCXTM geometry onto the pressurized, stretched micro-CT geometry. The output of the morphing model was a structural FEM simulation where the output strain distribution represents an estimation of the wall deformation, not only due to the pressurization, but also due to the local axial stretch field. The morphing model also included minor branches and a mouse-specific wall thickness. A sensitivity study was then performed to assess the influence of each of these novel features on the outcome of the simulations. The results were supported by comparing the computed hotspots of principal strain to hotspots of early vascular damage as detected on PCXTM. Non-uniform axial stretch, non-uniform wall thickness and minor subcostal arteries significantly alter the locations of calculated hotspots of maximal principal strain. Even if experimental data on these features are often not available in clinical practice, one should be aware of the important implications that simplifications in the model might have on the final simulated result.







Similar content being viewed by others
References
Antiga, L., and D. A. Steinman. Robust and objective decomposition and mapping of bifurcating vessels. IEEE Trans. Med. Imaging 23(6):704–713, 2004.
Antiga, L., M. Piccinelli, L. Botti, B. Ene-Iordache, A. Remuzzi, and D. A. Steinman. An image-based modeling framework for patient-specific computational hemodynamics. Med. Biol. Eng. Comput. 46(11):1097, 2008.
Avril, S., P. Badel, M. Gabr, M. A. Sutton, and S. M. Lessner. Biomechanics of porcine renal arteries and role of axial stretch. J. Biomech. Eng. 135(8):081007, 2013.
Bersi, M., J. Ferruzzi, J. Eberth, R. Gleason, Jr., and J. Humphrey. Consistent biomechanical phenotyping of common carotid arteries from seven genetic, pharmacological, and surgical mouse models. Ann. Biomed. Eng. 42(6):1207–1223, 2014.
Bols, J., L. Taelman, G. De Santis, J. Degroote, B. Verhegghe, P. Segers, and J. Vierendeels. Unstructured hexahedral mesh generation of complex vascular trees using a multi-block grid-based approach. Comput. Methods Biomech. Biomed. Eng. 19(6):663–672, 2016.
Bond, A. R., C.-W. Ni, H. Jo, and P. D. Weinberg. Intimal cushions and endothelial nuclear elongation around mouse aortic branches and their spatial correspondence with patterns of lipid deposition. Am. J. Physiol. Heart Circ. Physiol. 298(2):H536–H544, 2010.
Collins, M., M. Bersi, E. Wilson, and J. Humphrey. Mechanical properties of suprarenal and infrarenal abdominal aorta: implications for mouse models of aneurysms. Med. Eng. Phys. 33(10):1262–1269, 2011.
Conlisk, N., A. J. Geers, O. M. McBride, D. E. Newby, and P. R. Hoskins. Patient-specific modelling of abdominal aortic aneurysms: the influence of wall thickness on predicted clinical outcomes. Med. Eng. Phys. 38(6):526–537, 2016.
Daugherty, A., and L. A. Cassis. Mouse models of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 24(3):429–434, 2004.
Daugherty, A., M. W. Manning, and L. A. Cassis. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Investig. 105(11):1605–1612, 2000.
Dua, M. M., and R. L. Dalman. Hemodynamic influences on abdominal aortic aneurysm disease: application of biomechanics to aneurysm pathophysiology. Vasc. Pharmacol. 53(1):11–21, 2010.
Feintuch, A., P. Ruengsakulrach, A. Lin, J. Zhang, Y.-Q. Zhou, J. Bishop, et al. Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling. Am. J. Physiol. Heart Circ. Physiol. 292(2):H884–H892, 2007.
Figueroa, C. A., S. Baek, C. A. Taylor, and J. D. Humphrey. A computational framework for fluid–solid-growth modeling in cardiovascular simulations. Comput. Methods Appl. Mech. Eng. 198(45):3583–3602, 2009.
Friedman, J. H., J. L. Bentley, and R. A. Finkel. An algorithm for finding best matches in logarithmic expected time. ACM Trans. Math. Softw. 3(3):209–226, 1977.
Gamble, G., B. Beaumont, H. Smith, J. Zorn, G. Sanders, M. Merrilees, and N. Sharpe. B-mode ultrasound images of the carotid artery wall: correlation of ultrasound with histological measurements. Atherosclerosis 102(2):163–173, 1993.
Gasser, T. C., M. Auer, F. Labruto, J. Swedenborg, and J. Roy. Biomechanical rupture risk assessment of abdominal aortic aneurysms: model complexity versus predictability of finite element simulations. Eur. J. Vasc. Endovasc. Surg. 40(2):176–185, 2010.
Guo, X., and G. S. Kassab. Variation of mechanical properties along the length of the aorta in C57bl/6 mice. Am. J. Physiol. Heart Circ. Physiol. 285(6):H2614–H2622, 2003.
Haker, S., S. Angenent, A. Tannenbaurn, and R. Kikinis. Nondistorting flattening maps and the 3-D visualization of colon CT images. IEEE Trans. Med. Imaging 19(7):665–670, 2000.
Holzapfel, G. A., T. C. Gasser, and R. W. Ogden. A new constitutive framework for arterial wall mechanics and a comparative study of material models. Cardiovasc. Soft Tissue Mech. 61:1–48, 2001.
Humphrey, J. D., and G. A. Holzapfel. Mechanics, mechanobiology, and modeling of human abdominal aorta and aneurysms. J. Biomech. 45(5):805–814, 2012.
Humphrey, J., J. Eberth, W. Dye, and R. Gleason. Fundamental role of axial stress in compensatory adaptations by arteries. J. Biomech. 42(1):1–8, 2009.
Kim, J., and S. Baek. Circumferential variations of mechanical behavior of the porcine thoracic aorta during the inflation test. J. Biomech. 44(10):1941–1947, 2011.
Nakashima, Y., A. S. Plump, E. W. Raines, J. L. Breslow, and R. Ross. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. Vasc. Biol. 14(1):133–140, 1994.
Raaz, U., A. M. Zöllner, I. N. Schellinger, R. Toh, F. Nakagami, M. Brandt, et al. Segmental aortic stiffening contributes to experimental abdominal aortic aneurysm development. Circulation 2015. doi:10.1161/CIRCULATIONAHA.114.012377.
Raut, S. S., A. Jana, V. De Oliveira, S. C. Muluk, and E. A. Finol. The importance of patient-specific regionally varying wall thickness in abdominal aortic aneurysm biomechanics. J. Biomech. Eng. 135(8):081010, 2013.
Reymond, P., P. Crosetto, S. Deparis, A. Quarteroni, and N. Stergiopulos. Physiological simulation of blood flow in the aorta: comparison of hemodynamic indices as predicted by 3-D FSI, 3-D rigid wall and 1-D models. Med. Eng. Phys. 35(6):784–791, 2013.
Saraff, K., F. Babamusta, L. A. Cassis, and A. Daugherty. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 23(9):1621–1626, 2003.
Shang, E. K., D. P. Nathan, E. Y. Woo, R. M. Fairman, G. J. Wang, R. C. Gorman, and B. M. Jackson. Local wall thickness in finite element models improves prediction of abdominal aortic aneurysm growth. J. Vasc. Surg. 61(1):217–223, 2015.
Stampanoni, M., G. Borchert, P. Wyss, R. Abela, B. Patterson, S. Hunt, and P. Ruegsegger. High resolution X-ray detector for synchrotron-based microtomography. Nucl. Instrum. Methods Phys. Res. A 491(1):291–301, 2002.
Trachet, B., M. Renard, G. De Santis, S. Staelens, J. De Backer, L. Antiga, and P. Segers. An integrated framework to quantitatively link mouse-specific hemodynamics to aneurysm formation in angiotensin II-infused ApoE−/− mice. Ann. Biomed. Eng. 39(9):2430–2444, 2011.
Trachet, B., J. Bols, J. Degroote, B. Verhegghe, N. Stergiopulos, J. Vierendeels, and P. Segers. An animal-specific FSI model of the abdominal aorta in anesthetized mice. Ann. Biomed. Eng. 43(6):1298–1309, 2015.
Trachet, B., R. A. Fraga-Silva, A. Piersigilli, A. Tedgui, J. Sordet-Dessimoz, A. Astolfo, and N. Stergiopulos. Dissecting abdominal aortic aneurysm in Ang II-infused mice: suprarenal branch ruptures and apparent luminal dilatation. Cardiovasc. Res. 105(2):213–222, 2015.
Trachet, B., A. Piersigilli, L. Aslanidou, R. A. Fraga-Silva, J. Sordet-Dessimoz, P. Villanueva-Perez, and P. Segers. Angiotensin II infusion into ApoE−/− mice: a model for aortic dissection rather than abdominal aortic aneurysm? Cardiovasc. Res. 113(10):1230–1242, 2017.
Vincent, P., A. Plata, A. Hunt, P. Weinberg, and S. Sherwin. Blood flow in the rabbit aortic arch and descending thoracic aorta. J. R. Soc. Interface 8(65):1708–1719, 2011.
Vorp, D. A. Biomechanics of abdominal aortic aneurysm. J. Biomech. 40(9):1887–1902, 2007.
Voß, S., S. Glaßer, T. Hoffmann, O. Beuing, S. Weigand, K. Jachau, et al. Fluid–structure simulations of a ruptured intracranial aneurysm: constant versus patient-specific wall thickness. Comput. Math. Methods Med. 2016. doi:10.1155/2016/9854539.
Wehrl, H. F., I. Bezrukov, S. Wiehr, M. Lehnhoff, K. Fuchs, J. G. Mannheim, and B. J. Pichler. Assessment of murine brain tissue shrinkage caused by different histological fixatives using magnetic resonance and computed tomography imaging. Histol. Histopathol. 30(5):601–613, 2015.
Wilson, J., S. Baek, and J. Humphrey. Importance of initial aortic properties on the evolving regional anisotropy, stiffness and wall thickness of human abdominal aortic aneurysms. J. R. Soc. Interface 2012. doi:10.1098/rsif.2012.0097.
Wilson, J. S., M. R. Bersi, G. Li, and J. D. Humphrey. Correlation of wall microstructure and heterogeneous distributions of strain in evolving murine abdominal aortic aneurysms. Cardiovasc. Eng. Technol. 8(2):1–12, 2017.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferraro, M., Trachet, B., Aslanidou, L. et al. Should We Ignore What We Cannot Measure? How Non-Uniform Stretch, Non-Uniform Wall Thickness and Minor Side Branches Affect Computational Aortic Biomechanics in Mice. Ann Biomed Eng 46, 159–170 (2018). https://doi.org/10.1007/s10439-017-1945-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-017-1945-y