Abstract
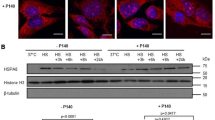
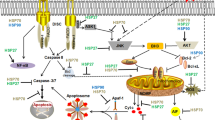
AlphaB-crystallin homology, heat stress induction and chaperone activity suggested that a previously encloned gene product is a novel small heat shock protein (Hsp16.2). Suppression of Hsp16.2 by siRNA sensitized cells to hydrogen peroxide or taxol induced cell-death. Over-expressing of Hsp16.2 protected cells against stress stimuli by inhibiting cytochrome c release from the mitochondria, nuclear translocation of AIF and endonuclease G, and caspase 3 activation. Recombinant Hsp16.2 protected mitochondrial membrane potential against calcium induced collapse in vitro indicating that Hsp16.2 stabilizes mitochondrial membrane systems. Hsp16.2 formed self-aggregates and bound to Hsp90. Inhibition of Hsp90 by geldanamycin diminished the cytoprotective effect of Hsp16.2 indicating that this effect was Hsp90-mediated. Hsp16.2 over-expression increased lipid rafts formation as demonstrated by increased cell surface labeling with fluorescent cholera toxin B, and increased Akt phosphorylation. The inhibition of PI-3-kinase—Akt pathway by LY-294002 or wortmannin significantly decreased the protective effect of the Hsp16.2. These data indicate that the over-expression of Hsp16.2 inhibits cell death via the stabilization of mitochondrial membrane system, activation of Hsp90, stabilization of lipid rafts and by the activation of PI-3-kinase—Akt cytoprotective pathway.
Similar content being viewed by others
References
Sun Y, MacRae TH (2005) The small heat shock proteins and their role in human disease. FEBS J 272:2613–627
Parcellier A, Schmitt E, Brunet M et al (2005) Small heat shock proteins Hsp27 and alphaB-crystallin: cytoprotective and oncogenic functions. Antioxid Redox Signal 7:404–13
Arrigo AP (2005) In search of the molecular mechanism by which small stress proteins counteract apoptosis during cellular differentiation. J Cell Biochem 94:241–46
Arrigo AP, Paul C, Ducasse C et al (2002) Small stress proteins: modulation of intracellular redox state and protection against oxidative stress. Prog Mol Subcell Biol 28:171–84
Kamradt MC, Lu M, Werner M et al (2005) The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem 280:11059–1066
Schepers H, Geugien M, Van Der Toorn M et al (2005) HSP27 protects AML cells against VP-16-induced apoptosis through modulation of p38 and c-Jun. Exp Hematol 33:660–70
Nakagomi S, Suzuki Y, Namikawa K et al (2003) Expression of the activating transcription factor 3 prevents c-Jun N-terminal kinase-induced neuronal death by promoting heat shock protein 27 expression and Akt activation. J Neurosci 23:5187–196
Mehlen P, Preville X, Chareyron P et al (1995) Constitutive expression of human hsp27, Drosophila hsp27, or human αB-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol 154:363–74
Kamradt MC, Chen F, Cryns VL (2001) The small heat shock protein αB-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem 276:16059–6063
Kamradt MC, Chen F, Sam S et al (2002) The small heat shock protein αB-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem 277:38731–8736
Mao YW, Liu JP, Xiang H et al (2004) Human αA- and αB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ 11:512–26
Sreedhar AS, Csermely P (2004) Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol Ther 101:227–57
Tsuruo T, Naito M, Tomida A et al (2003) Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci 94:15–1
Lee SA, Ndisang D, Patel C et al (2005) Expression of the Brn-3b transcription factor correlates with expression of Hsp-27 in breast cancer biopsies and is required for maximal activation of the Hsp-27 promoter. Cancer Res 65:3072–080
Kang SH, Fung MA, Gandour-Edwards R et al (2004) Heat shock protein 27 is expressed in normal and malignant human melanocytes in vivo. J Cutan Pathol 31:665–71
Rocchi P, So A, Kojima S et al (2004) Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res 64:6595–602
Kappe G, Franck E, Verschuure P et al (2003) The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones 8:53–1
Altschul SF, Madden TL, Schäffer AA et al (1997) BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–402
Pruitt KD, Maglott DR (2001) RefSeq and LocusLink: NCBI gene-centered resources. Nucleic Acids Res 29:137–40
Tapodi A, Debreceni B, Hanto K et al (2005) Pivotal role of Akt activation in mitochondrial protection and cell survival by poly(ADP-ribose)polymerase-1 inhibition in oxidative stress. J Biol Chem 280:35767–5775
Haslbeck M, Walke S, Stromer T et al (1999) Hsp26: a temperature-regulated chaperonee. EMBO J 18:6744–751
Jakob U, Lilie H, Meyer I et al (1995) Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase. Implications for heat shock in vivo. J Biol Chem 270:7288–294
Sims NR (1990) Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem 55:698–07
Shevchenko A, Wilm M, Vorm O et al (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68:850–58
Clauser KR, Baker PR, Burlingame AL (1999) Role of accurate mass measurement (+/− 10 ppm) in protein identification strategies employing MS or MS/MS and database searching. Anal Chem 71:2871–882
Buchner J, Grallert H, Jakob U (1998) Analysis of chaperonee function using citrate synthase as nonnative substrate protein. Methods Enzymol 290:323–38
Reers M, Smith TW, Chen LB (1991) J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 30:4480–486
Sreedhar AS, Mihaly K, Pato B et al (2003) Hsp90 inhibition accelerates cell lysis. Anti-Hsp90 ribozyme reveals a complex mechanism of Hsp90 inhibitors involving both superoxide- and Hsp90-dependent events. J Biol Chem 278:35231–5240
Chen S, Bawa D, Besshoh S et al (2005) Association of heat shock proteins and neuronal membrane components with lipid rafts from the rat brain. J Neurosci Res 81:522–29
Waheed AA, Jones TL (2002) Hsp90 interactions and acylation target the G protein Galpha 12 but not Galpha 13 to lipid rafts. J Biol Chem 277:32409–2412
Soti C, Csermely P (1998) Molecular chaperonees in the etiology and therapy of cancer. Pathol Oncol Res 4:316–21
Jolly C, Morimoto RI (2000) Role of the heat shock response and molecular chaperonees in oncogenesis and cell death. J Nat Cancer Inst 92:1564–572
Sun Y, Mansour M, Crack JA et al (2004) Oligomerization, chaperonee activity, and nuclear localization of p26, a small heat shock protein from Artemia franciscana. J Biol Chem 279:39999–0006
Varbiro G, Toth A, Tapodi A et al (2003) Protective effect of amiodarone but not N-desethylamiodarone on postischemic hearts through the inhibition of mitochondrial permeability transition. J Pharmacol Exp Ther 307:615–25
Mukai M, Kusama T, Hamanaka Y et al (2005) Cross talk between apoptosis and invasion signaling in cancer cells through caspase-3 activation. Cancer Res 65:121–25
Triantafilou M, Miyake K, Golenbock DT et al (2002) Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci 115:2603–611
Jain S, Li Y, Kumar A et al (2005) Transcriptional signaling from membrane raft-associated glucocorticoid receptor. Biochem Biophys Res Commun 336:3–
Shah M, Patel K, Fried VA et al (2002) Interactions of STAT3 with caveolin-1 and heat shock protein 90 in plasma membrane raft and cytosolic complexes. Preservation of cytokine signaling during fever. J Biol Chem 277:45662–5669
Hill MM, Feng J, Hemmings BA (2002) Identification of a plasma membrane Raft-associated PKB Ser473 kinase activity that is distinct from ILK and PDK1. Curr Biol 12:1251–255
Plas DR, Thompson CB (2005) Akt-dependent transformation: there is more to growth than just surviving. Oncogene 24:7435–442
Cheng JQ, Lindsley CW, Cheng GZ et al (2005) The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene 24:7482–492
Neckers L, Ivy SP (2003) Heat shock protein 90. Curr Opin Oncol 15:419–24
Brazil DP, Yang ZZ, Hemmings BA (2004) Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci 29:233–42
Brognard J, Clark AS, Ni Y et al (2001) Akt/Protein Kinase B is constitutively active in non-small cell cung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res 61:3986–997
Ringel MD, Hayre N, Saito J et al (2001) Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res 61:6105–111
Tanno S, Yanagawa N, Habiro A et al (2004) Serine/Threonine kinase AKT is frequently activated in human bile duct cancer and is associated with increased radioresistance. Cancer Res 64:3486–490
Yang L, Dan HC, Sun M et al (2004) Akt/Protein Kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with atitumor activity in cancer cells overexpressing Akt. Cancer Res 64:4394–399
Elhyany S, Assa-Kunik E, Tsory S et al (2004) The integrity of cholesterol-enriched microdomains is essential for the constitutive high activity of protein kinase B in tumour cells. Biochem Soc Trans 32:837–39
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bellyei, S., Szigeti, A., Boronkai, A. et al. Inhibition of cell death by a novel 16.2 kD heat shock protein predominantly via Hsp90 mediated lipid rafts stabilization and Akt activation pathway. Apoptosis 12, 97–112 (2007). https://doi.org/10.1007/s10495-006-0486-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-0486-x