Abstract
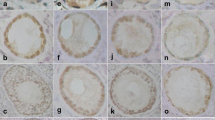
One of the characteristics of polycystic ovary syndrome (PCOS) is the presence of cystic follicles in various stages of growth and atresia, the latter of which is known to be the result of apoptosis and tissue remodeling. To further investigate the process of follicular atresia, we compared ovarian expression and localization of Fas, Fas ligand (FasL), casapse-8 and membrane-type1 matrix metalloproteinase (MT1-MMP) in rats treated with dehydroepiandrosterone (DHEA) as a model of PCOS, and in control rats. We found that the numbers of TdT-mediated dUTP-biotin nick end-labeling (TUNEL)-positive follicles were significantly higher in ovaries from PCOS rats than in those from control rats (P < 0.05), as were ovarian levels of FasL mRNA and protein, processed caspase-8 protein and MT1-MMP mRNA. Correspondingly, we also observed an increase in the level of MTI-MMP catalytic activity and a decrease in the level of pro-caspase-8 protein. In addition, immunohistochemical analyses showed that MT1-MMP and FasL co-localize with TUNEL-positive apoptotic granulosa cells within atretic follicles of PCOS ovaries. Our results suggest that under the PCOS-like conditions induced by DHEA, the Fas/FasL/Caspase-8 (death receptor dependent) pathway is pivotal for follicular atresia, and that increased levels of MT1-MMP likely play an important role in tissue remodeling during structural luteolysis.
Similar content being viewed by others
References
Sommers SC, Wadman PJ (1956) Pathogenesis of polycystic ovaries. Am J Obstet Gynecol 72:160–169
Singh KB (2005) Persistent estrus rat models of polycystic ovary disease: an update. Fertil Steril 84 Suppl 2:1228–1234
Ruiz A, Aguilar R, Tebar AM, Gaytan F (1996) RU486-treated rats show endocrine and morphological responses to therapies analogous to responses of women with polycystic ovary syndrome treated with similar therapies. Biol Reprod 55:1284–1291
Jones HM, Vernon MW, Rush ME (1987) Systematic studies invalidate the neonatally androgenized rat as a model for polycystic ovary disease. Biol Reprod 36:1253–1265
Beloosesky R, Gold R, Almog B (2004) Induction of polycystic ovary by testosterone in immature female rats: Modulation of apoptosis and attenuation of glucose/insulin ratio. Int J Mol Med 14:207–215
Mahesh VB (1966) Androgen secretion in the Stein-Leventhal syndrome. Proc R Soc Med 59:1289–1291
Greenblatt RB, Mahesh VB (1974) Some new thoughts on the Stein-Leventhal syndrome. J Reprod Med 13:85–88
Roy S, Mahesh VB, Greenblatt RB (1962) Effect of dehydroepiandrosterone and delta4-androstenedione on the reproductive organs of female rats: production of cystic changes in the ovary. Nature 196:42–43
Mahesh VB, Mills TM, Bagnell CA, Conway BA (1987) Animal models for study of polycystic ovaries and ovarian atresia. Adv Exp Med Biol 219:237–257
Anderson E, Lee MT, Lee GY (1992) Cystogenesis of the ovarian antral follicle of the rat: ultrastructural changes and hormonal profile following the administration of dehydroepiandrosterone. Anat Rec 234:359–382
Anderson E, Lee GY, O'Brien K (1997) Polycystic ovarian condition in the dehydroepiandrosterone-treated rat model: hyperandrogenism and the resumption of meiosis are major initial events associated with cystogenesis of antral follicles. Anat Rec 249:44–53
Anderson E, Lee GY (1997) The polycystic ovarian (PCO) condition: apoptosis and epithelialization of the ovarian antral follicles are aspects of cystogenesis in the dehydroepiandrosterone (DHEA)-treated rat model. Tissue Cell 29:171–189
Anderson E, Lee GY (1996) The effects of dehydroepiandrosterone (DHEA) and its metabolites on the polycystic ovarian condition (PCO): cystogenic changes of rat granulosa cells in vitro. Tissue Cell 28:673–685
Henmi H, Endo T, Nagasawa K et al (2001) Lysyl oxidase and MMP-2 expression in dehydroepiandrosterone-induced polycystic ovary in rats. Biol Reprod 64:157–162
Lee MT, Anderson E, Lee GY (1991) Change in ovarian morphology and serum hormones in the rat after treatment with dehydroepiandrosterone. Anat Rec 231:185–192
Parker CR, Mahesh VB (1976) Interrelationship between excessive levels of circulating androgens in blood and ovulatory failure. J Reprod Med 17:75–90
Hsueh AJ, Billig H, Tsafriri A (1994) Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev 15:707–724
Ju ST, Panka DJ, Cui H (1995) Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 373:444–448
Kim JM, Boone DL, Auyeung A, Tsang BK (1998) Granulosa cell apoptosis induced at the penultimate stage of follicular development is associated with increased levels of Fas and Fas ligand in the rat ovary. Biol Reprod 58:1170–1176
Roughton SA, Lareu RR, Bittles AH, Dharmarajan AM (1999) Fas and Fas ligand messenger ribonucleic acid and protein expression in the rat corpus luteum during apoptosis-mediated luteolysis. Biol Reprod 60:797–804
Hakuno N, Koji T, Yano T et al (1996) Fas/APO-1/CD95 system as a mediator of granulosa cell apoptosis in ovarian follicle atresia. Endocrinology 137:1938–1948
Sakamaki K, Yoshida H, Nishimura Y, Nishikawa S, Manabe N, Yonehara S (1997) Involvement of Fas antigen in ovarian follicular atresia and luteolysis. Mol Reprod Dev 47:11–18
Manabe N, Goto Y, Matusda-Minehata F et al (2004) Regulation mechanism of selective atresia in porcine follicles: regulation of granulosa cell apoptosis during atresia. J Reprod Dev 50:493–514
Cataldo NA, Dumesic DA, Goldsmith PC, Jaffe RB (2000) Immunolocalization of Fas and Fas ligand in the ovaries of women with polycystic ovary syndrome: relationship to apoptosis. Hum Reprod 15:1889–1897
Tsafriri A (1995) Ovulation as a tissue remodelling process. Proteolysis and cumulus expansion. Adv Exp Med Biol 377:121–140
Ny T, Wahlberg P, Brandstrom IJ (2002) Matrix remodeling in the ovary: regulation and functional role of the plasminogen activator and matrix metalloproteinase systems. Mol Cell Endocrinol 187:29–38
Endo T, Aten RF, Wang F, Behrman HR (1993) Coordinate induction and activation of metalloproteinase and ascorbate depletion in structural luteolysis. Endocrinology 133:690–698
Liu K, Olofsson JI, Wahlberg P, Ny T (1999) Distinct expression of gelatinase A [matrix metalloproteinase (MMP)-2], collagenase-3 (MMP-13), membrane type MMP 1 (MMP-14), and tissue inhibitor of MMPs type 1 mediated by physiological signals during formation and regression of the rat corpus luteum. Endocrinology 140:5330–5338
Goto T, Endo T, Henmi H et al (1999) Gonadotropin-releasing hormone agonist has the ability to induce increased matrix metalloproteinase (MMP)-2 and membrane type 1-MMP expression in corpora lutea, and structural luteolysis in rats. J Endocrinol 161:393–402
Manase K, Endo T, Henmi H et al (2002) The significance of membrane type 1 metalloproteinase in structural involution of human corpora lutea. Mol Hum Reprod 8:742–749
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Sato H, Kida Y, Mai M et al (1992) Expression of genes encoding type IV collagen-degrading metalloproteinases and tissue inhibitors of metalloproteinases in various human tumor cells. Oncogene 7:77–83
Yamazaki K, Endo T, Kitajima Y et al (2006) Elevation of both Cyclooxygenase-2 and Prostaglandin E(2) Receptor EP3 expressions in rat placenta after uterine artery ischemia-reperfusion. Placenta 27:395–401
KitajimaY, Endo T, Nagasawa K et al (2006) Hyperstimulation and a gonadotropin-releasing hormone agonist modulate ovarian vascular permeability by altering expression of the tight junction protein claudin-5. Endocrinology 47:694–699
Hamm ML, Bhat GK, Thompson WE, Mann DR (2004) Folliculogenesis is impaired and granulosa cell apoptosis is increased in leptin-deficient mice. Biol Reprod 71:66–72
Nagata S (1997) Apoptosis by death factor. Cell 88:355–365
Kuranaga E, Kanuka H, Bannai M, Suzuki M, Nishihara M, Takahashi M (1999) Fas/Fas ligand system in prolactin-induced apoptosis in rat corpus luteum: possible role of luteal immune cells. Biochem Biophys Res Commun 260:167–173
Syed V, Ho SM (2003) Progesterone-induced apoptosis in immortalized normal and malignant human ovarian surface epithelial cells involves enhanced expression of FasL. Oncogene 22:6883–6890
Muzio M, Chinnaiyan AM, Kischkel FC et al (1996) FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death–inducing signaling complex. Cell 85:817–827
Medema JP, Scaffidi C, Kischkel FC et al (1997) FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). Embo J 16:2794–2804
Navarro A, Torrejon R, Bandez MJ, Lopez-Cepero JM, Boveris A (2005) Mitochondrial function and mitochondria-induced apoptosis in an overstimulated rat ovarian cycle. Am J Physiol Endocrinol Metab 289:E1101–E1109
Harayama T, Ohuchi E, Aoki T, Sato H, Seiki M, Okada Y (1999) Shedding of membrane type 1 matrix metalloproteinase in a human breast carcinoma cell line. Jpn J Cancer Res 90:942–950
Holmbeck K, Bianco P, Chrysovergis K, Yamada S, Birkedal-Hansen H (2003) MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: a critical process in skeletal growth. J Cell Biol 163:661–671
d'Ortho MP, Will H, Atkinson S et al (1997) Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem 250:751–757
Polette M, Birembaut P (1998) Membrane-type metalloproteinases in tumor invasion. Int J Biochem Cell Biol 30:1195–1202
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Honnma, H., Endo, T., Henmi, H. et al. Altered expression of Fas/Fas ligand/caspase 8 and membrane type 1-matrix metalloproteinase in atretic follicles within dehydroepiandrosterone-induced polycystic ovaries in rats. Apoptosis 11, 1525–1533 (2006). https://doi.org/10.1007/s10495-006-9148-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-9148-2