Abstract
TRAIL, a ligand of the TNFα family, induces upon binding to its pro-death receptors TRAIL-R1/DR4 and TRAIL-R2/DR5 the apoptosis of cancer cells. Activated receptors incite the formation of the Death-Inducing Signaling Complex followed by the activation of the downstream apoptotic signaling. TRAIL-induced apoptosis is regulated at multiple levels, one of them being the presence and relative number of TRAIL pro- and anti-apoptotic receptors on the cytoplasmic membrane. In a yeast two-hybrid search for proteins that interact with the intracellular part (ICP) of DR4, we picked ARAP1, an adapter protein with ArfGAP and RhoGAP activities. In yeast, DR4(ICP) interacts with the alternatively spliced ARAP1 lacking 11 amino acids from the PH5 domain. Transfected ARAP1 co-precipitates with DR4 and co-localizes with it in the endoplasmic reticulum/Golgi, at the cytoplasmic membrane and in early endosomes of TRAIL-treated cells. ARAP1 knockdown significantly compromises the localization of DR4 at the cell surface of several tumor cell lines and slows down their TRAIL-induced death. ARAP1 overexpressed in HEL cells does not affect their TRAIL-induced apoptosis or the membrane localization of DR4, but it enhances the cell-surface presentation of phosphatidyl serine. Our data indicate that ARAP1 is likely involved in the regulation of the cell-specific trafficking of DR4 and might thus affect the efficacy of TRAIL-induced apoptosis.






Similar content being viewed by others
Abbreviations
- TNF:
-
Tumor necrosis factor
- TRAIL/Apo2L:
-
TNF-related apoptosis-inducing ligand
- Arf:
-
ADP-ribosylation factor small GTPase
- ARAP1:
-
ArfGAP, RhoGAP, Ankyrin repeats and pleckstrin homology (PH) domains containing protein
- DD:
-
Death domain
- DISC:
-
Death-inducing signaling complex
- ER:
-
Endoplasmic reticulum
- GAP:
-
GTPase-activating protein
- IAP:
-
Inhibitor of apoptosis
- MFI:
-
Median fluorescence intensity
- MMP:
-
Mitochondrial membrane permeabilization
- PARP:
-
Poly-ADP ribose polymerase
- SiRNA:
-
Small inhibitory RNA
- TGN:
-
Trans-Golgi network
References
Wiley SR, Schooley K, Smolak PJ et al (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3:673–682
Walczak H, Krammer PH (2000) The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res 256:58–66
Ashkenazi A, Pai RC, Fong S et al (1999) Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 104:155–162
Walczak H, Miller RE, Ariail K et al (1999) Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 5:157–163
Pan G, O’Rourke K, Chinnaiyan AM et al (1997) The receptor for the cytotoxic ligand TRAIL. Science 276:111–113
Walczak H, Degli-Esposti MA, Johnson RS et al (1997) TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. Embo J 16:5386–5397
Degli-Esposti MA, Smolak PJ, Walczak H et al (1997) Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med 186:1165–1170
Degli-Esposti MA, Dougall WC, Smolak PJ et al (1997) The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 7:813–820
Emery JG, McDonnell P, Burke MB et al (1998) Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem 273:14363–14367
Chaudhari BR, Murphy RF, Agrawal DK (2006) Following the TRAIL to apoptosis. Immunol Res 35:249–262
Tartaglia LA, Ayres TM, Wong GH, Goeddel DV (1993) A novel domain within the 55 kd TNF receptor signals cell death. Cell 74:845–853
Kischkel FC, Hellbardt S, Behrmann I et al (1995) Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. Embo J 14:5579–5588
Sprick MR, Weigand MA, Rieser E et al (2000) FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12:599–609
Salvesen GS, Dixit VM (1999) Caspase activation: the induced-proximity model. Proc Natl Acad Sci USA 96:10964–10967
Barnhart BC, Alappat EC, Peter ME (2003) The CD95 type I/type II model. Semin Immunol 15:185–193
Luo X, Budihardjo I, Zou H et al (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481–490
Aggarwal BB, Bhardwaj U, Takada Y (2004) Regulation of TRAIL-induced apoptosis by ectopic expression of antiapoptotic factors. Vitam Horm 67:453–483
Kimberley FC, Screaton GR (2004) Following a TRAIL: update on a ligand and its five receptors. Cell Res 14:359–372
Zhang XD, Franco AV, Nguyen T et al (2000) Differential localization and regulation of death and decoy receptors for TNF-related apoptosis-inducing ligand (TRAIL) in human melanoma cells. J Immunol 164:3961–3970
Bennett M, Macdonald K, Chan SW et al (1998) Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science 282:290–293
Jones SJ, Ledgerwood EC, Prins JB et al (1999) TNF recruits TRADD to the plasma membrane but not the trans-Golgi network, the principal subcellular location of TNF-R1. J Immunol 162:1042–1048
Storey H, Stewart A, Vandenabeele P, Luzio JP (2002) The p55 tumour necrosis factor receptor TNFR1 contains a trans-Golgi network localization signal in the C-terminal region of its cytoplasmic tail. Biochem J 366:15–22
Nie Z, Hirsch DS, Luo R et al (2006) A BAR domain in the N terminus of the Arf GAP ASAP1 affects membrane structure and trafficking of epidermal growth factor receptor. Curr Biol 16:130–139
Randazzo PA, Nie Z, Miura K, Hsu VW (2000) Molecular aspects of the cellular activities of ADP-ribosylation factors. Sci STKE 2000:RE1
Randazzo PA, Hirsch DS (2004) Arf GAPs: multifunctional proteins that regulate membrane traffic and actin remodelling. Cell Signal 16:401–413
Dai J, Li J, Bos E et al (2004) ACAP1 promotes endocytic recycling by recognizing recycling sorting signals. Dev Cell 7:771–776
Majoul I, Straub M, Hell SW et al (2001) KDEL-cargo regulates interactions between proteins involved in COPI vesicle traffic: measurements in living cells using FRET. Dev Cell 1:139–153
Miura K, Jacques KM, Stauffer S et al (2002) ARAP1: a point of convergence for Arf and Rho signaling. Mol Cell 9:109–119
Plasilova M, Zivny J, Jelinek J et al (2002) TRAIL (Apo2L) suppresses growth of primary human leukemia and myelodysplasia progenitors. Leukemia 16:67–73
Matsuda D, Nakayama Y, Horimoto S et al (2006) Involvement of Golgi-associated Lyn tyrosine kinase in the translocation of annexin II to the endoplasmic reticulum under oxidative stress. Exp Cell Res 312:1205–1217
Schaefer U, Voloshanenko O, Willen D, Walczak H (2007) TRAIL: a multifunctional cytokine. Front Biosci 12:3813–3824
Thorburn A (2007) Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) pathway signaling. J Thorac Oncol 2:461–465
Lin Y, Devin A, Cook A et al (2000) The death domain kinase RIP is essential for TRAIL (Apo2L)-induced activation of IkappaB kinase and c-Jun N-terminal kinase. Mol Cell Biol 20:6638–6645
Meurette O, Rebillard A, Huc L et al (2007) TRAIL induces receptor-interacting protein 1-dependent and caspase-dependent necrosis-like cell death under acidic extracellular conditions. Cancer Res 67:218–226
Mulherkar N, Prasad KV, Prabhakar BS (2007) MADD/DENN splice variant of the IG20 gene is a negative regulator of caspase-8 activation. Knockdown enhances TRAIL-induced apoptosis of cancer cells. J Biol Chem 282:11715–11721
Ramaswamy M, Efimova EV, Martinez O et al (2004) IG20 (MADD splice variant-5), a proapoptotic protein, interacts with DR4/DR5 and enhances TRAIL-induced apoptosis by increasing recruitment of FADD and caspase-8 to the DISC. Oncogene 23:6083–6094
Brett TJ, Traub LM, Fremont DH (2002) Accessory protein recruitment motifs in clathrin-mediated endocytosis. Structure 10:797–809
Ritter B, Denisov AY, Philie J et al (2007) The NECAP PHear domain increases clathrin accessory protein binding potential. Embo J 26:4066–4077
Thomas LR, Johnson RL, Reed JC, Thorburn A (2004) The C-terminal tails of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas receptors have opposing functions in Fas-associated death domain (FADD) recruitment and can regulate agonist-specific mechanisms of receptor activation. J Biol Chem 279:52479–52486
Krugmann S, Anderson KE, Ridley SH et al (2002) Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol Cell 9:95–108
Elliott JI, Surprenant A, Marelli-Berg FM et al (2005) Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat Cell Biol 7:808–816
Smrz D, Draberova L, Draber P (2007) Non-apoptotic phosphatidylserine externalization induced by engagement of glycosylphosphatidylinositol-anchored proteins. J Biol Chem 282:10487–10497
Stacey TTI, Nie Z, Stewart A et al (2004) ARAP3 is transiently tyrosine phosphorylated in cells attaching to fibronectin and inhibits cell spreading in a RhoGAP-dependent manner. J Cell Sci 117:6071–6084
Krugmann S, Andrews S, Stephens L, Hawkins PT (2006) ARAP3 is essential for formation of lamellipodia after growth factor stimulation. J Cell Sci 119:425–432
Yoon HY, Miura K, Cuthbert EJ et al (2006) ARAP2 effects on the actin cytoskeleton are dependent on Arf6-specific GTPase-activating-protein activity and binding to RhoA-GTP. J Cell Sci 119:4650–4666
Kowanetz K, Husnjak K, Holler D et al (2004) CIN85 associates with multiple effectors controlling intracellular trafficking of epidermal growth factor receptors. Mol Biol Cell 15:3155–3166
Nie Z, Fei J, Premont RT, Randazzo PA (2005) The Arf GAPs AGAP1 and AGAP2 distinguish between the adaptor protein complexes AP-1 and AP-3. J Cell Sci 118:3555–3566
Jin Z, McDonald ER 3rd, Dicker DT, El-Deiry WS (2004) Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J Biol Chem 279:35829–35839
Ren YG, Wagner KW, Knee DA et al (2004) Differential regulation of the TRAIL death receptors DR4 and DR5 by the signal recognition particle. Mol Biol Cell 15:5064–5074
Ivanov VN, Ronai Z, Hei TK (2006) Opposite roles of FAP-1 and dynamin in the regulation of Fas (CD95) translocation to the cell surface and susceptibility to Fas ligand-mediated apoptosis. J Biol Chem 281:1840–1852
Sodeman T, Bronk SF, Roberts PJ et al (2000) Bile salts mediate hepatocyte apoptosis by increasing cell surface trafficking of Fas. Am J Physiol Gastrointest Liver Physiol 278:G992–999
Voelkel-Johnson C (2003) An antibody against DR4 (TRAIL-R1) in combination with doxorubicin selectively kills malignant but not normal prostate cells. Cancer Biol Ther 2:283–290
Acknowledgements
We are grateful to Drs. J. Tschopp, L. LoMuzio, P.A. Randazzo, S. Krugmann and K. Drbal for providing cells and reagents, and J. Dutt for critical reading of the manuscript. The project was supported by the Center for Molecular and Cellular Immunology 1M0506, the FP6 program LSHG-CT-2006-037278 and by the Institutional Grant AV0Z50520514.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary materials.
Suppl. Table 1
Sequences of the primers used for the generation of DR4 mutants and for RT-PCR or real-time PCR to detect the relative abundance of the splice variant ARAP1Δexon30. (EPS 403 kb)
Suppl. Fig. 1
ARAP1Dexon30 is the main splice variant expressed in human tumor-derived cell lines. (A) cDNAs prepared from different primary cells and cell lines were analyzed for their relative expression of ARAP1 splice variants (FL and Dexon30) by quantitative real-time PCR as described in Suppl. Materials and Methods. cDNAs prepared from HEK293 cells transfected either with ARAP1FL or ARAP1Δexon30 were used as a controls. Average means and standard errors from 3 independent experiments are shown. (B) Apa1 cleavage of exon 30 within the PCR-amplified 3′ end of ARAP1 (3350–4495; arrow) yields two fragments of 417 bp and 729 bp (asterisks); the PCR product (1146 bp) of ARAP1-CΔexon30 is not cleaved. 1,2—uncleaved and Apa I-digested PCR product of ARAP1-FL; 3,4—uncleaved and Apa I-digested PCR product of ARAP1 amplified from NCTC cDNA. Gene Ruler 1 kb DNA Ladder (Fermentas) was used as a size marker. (EPS 368 kb)
Suppl. Fig. 2
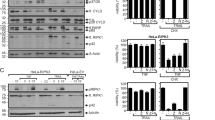
(A) Alignment of the first two a-helices in the death domains of the TNFR death receptors. Amino acids F377 and D378 in DR4/DR5 that were in DR4 mutated to proline and leucine, respectively, are shown in the box. First and second a-helices in Fas DD are underlined. (B) NCTC cells were treated either with TRAIL (200 ng/ml) for 20 and 50 min or pre-incubated with zVAD (20 μM final concentration) for 30 min prior to the TRAIL treatment. Cell lysates were precipitated with anti-DR4 monoclonal antibody and analyzed by Western blotting with the appropriate antibodies (Ips, immunoprecipitations; CLs, cell lysates). Filled arrow marks ARAP1(FL) and empty arrow processed form of ARAP1. (EPS 2497 kb)
Suppl. Fig. 3
Graphical representation of the relative cell surface expression of selected receptors on Saos-2, MG-63 and HCT116 cells. Relative representations of the averaged fluorescence medians for Saos-2, MG-63 and HCT116 cells from 4 independent experiments, together with the standard errors and statistical significance, are shown (* = P < 0.05). (EPS 487 kb)
Suppl. Fig. 4
Suppression of ARAP1 expression slows down TRAIL-induced apoptosis of MG-63 cells. MG-63 cells were grown in 24-well plates and transfected either with luciferase or with ARAP1 siRNA. Fifty-two hours after transfection, some of the cells transfected with luciferase siRNA were pre-incubated with anti-DR4 or anti-DR6 monoclonal antibodies antibody (at a final conc. of 10 mg/ml for 1 h) before the TRAIL treatment. Cells were treated with TRAIL (200 ng/ml) plus cycloheximide (10 ug/ml) for 0, 90, 120, 150 or 180 min, harvested and washed with ice-cold PBS. Cells were then fixed with ice-cold methanol, stained with FITC-conjugated anti-M30 antibody according to the manufacturer’s protocol and analyzed by flow cytometry. The abscissa above the histograms with the percentage indicated represents the percentage of M30-positive, apoptotic cells. (EPS 2757 kb)
Suppl. Fig. 5
ARAP1 co-fractionated with DR4 in the light membrane fractions of a sucrose-density gradient. NCTC cells were grown in 100 mm plates and transfected either with luciferase or with ARAP1 siRNA. Fifty-two hours after transfection, cells were lysed by hypotonic lysis and the postnuclear supernatants were subjected to sucrose-density gradient centrifugation. The gradient was split into 10 equal volume fractions from the top to the bottom. One tenth of each fraction together with the pellet was analyzed by Western blotting using anti-DR4 and ARAP-1 antibodies. The light membrane fractions (1–3) contain the plasma membrane. Arrows indicate the migration of DR4. (EPS 518 kb)
Below is the link to the electronic supplementary materials.
Rights and permissions
About this article
Cite this article
Šímová, Š., Klíma, M., Cermak, L. et al. Arf and Rho GAP adapter protein ARAP1 participates in the mobilization of TRAIL-R1/DR4 to the plasma membrane. Apoptosis 13, 423–436 (2008). https://doi.org/10.1007/s10495-007-0171-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-007-0171-8