Abstract
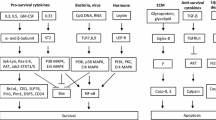
Apoptosis, the most common form of cell death, is a key mechanism in the build up and maintenance of both innate and adaptive immunity. Central to the apoptotic process is a family of intracellular cysteine proteases with aspartate-specificity, called caspases. Caspases are counter-regulated by multiple anti-apoptotic molecules, and the expression of the latter in leukocytes is largely dependent on survival factors. Therefore, the physiologic rates of apoptosis change under pathologic conditions. For instance, in inflammation, the expression of survival factors is usually elevated, resulting in increased cell survival and consequently in the accumulation of the involved immune cells. In many allergic diseases, eosinophil apoptosis is delayed contributing to both blood and tissue eosinophilia. Besides eosinophils, apoptosis of other leukocytes is also frequently prevented or delayed during allergic inflammatory processes. In contrast to inflammatory cells, accelerated cell death is often observed in epithelial cells, a mechanism, which amplifies or at least maintains allergic inflammation. In conclusion, deregulated cell death is a common phenomenon of allergic diseases that likely plays an important role in their pathogenesis. Whether the apoptosis is too little or too much depends on the cell type. In this review, we discuss the regulation of the lifespan of the participating leukocytes in allergic inflammatory responses.


Similar content being viewed by others
References
Asher MI, Montefort S, Bjorksten B et al (2006) Worldwide time trends in the prevalence and symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 368:733–743. doi:10.1016/S0140-6736(06)69283-0
Bradding P, Walls AF, Holgate ST (2006) The role of mast cells in the pathophysiology of asthma. J Allergy Clin Immunol 117:1277–1284. doi:10.1016/j.jaci.2006.02.039
Gibson PG, Allen CJ, Yang JP et al (1993) Intraepithelial mast cells in allergic and nonallergic asthma: assessment using bronchial brushings. Am Rev Respir Dis 148:80–86
Mekori YA, Oh CK, Metcalfe DD (1993) IL-3-dependent murine mast cells undergo apoptosis on removal of IL-3: prevention of apoptosis by c-kit ligand. J Immunol 151:3775–3784
Iemura A, Tsai M, Ando A, Wershil BK, Galli SJ (1994) The c-kit ligand, stem cell factor, promotes mast cell survival by suppressing apoptosis. Am J Pathol 144:321–328
Yanagida M, Fukamachi H, Ohgami K et al (1995) Effects of T-helper 2-type cytokines, interleukin-3 (IL-3), IL-4, IL-5, and IL-6 on the survival of cultured human mast cells. Blood 86:3705–3714
Shelburne CP, Ryan JJ (2001) The role of Th2 cytokines in mast cell homeostasis. Immunol Rev 179:82–93. doi:10.1034/j.1600-065X.2001.790109.x
Kitamura Y, Go S, Hatanaka K (1978) Decrease of mast cells in W/Wv mice and their increase by bone-marrow transplantation. Blood 52:447–452
Kitumara Y, Go S (1979) Decreased production of mast cells in S1/S1d anemic mice. Blood 53:492–497
Heinrich MC, Dooley DC, Freed AC et al (1993) Constitutive expression of steel factor gene by human stromal cells. Blood 82:771–783
Zhang S, Anderson DF, Bradding P et al (1998) Human mast cells express stem cell factor. J Pathol 186:59–66. doi:10.1002/(SICI)1096-9896(199809)186:1<59::AID-PATH140>3.0.CO;2-J
Wershil BK, Tsai M, Geissler EN, Zsebo KM, Galli SJ (1992) The rat c-kit ligand, stem cell factor, induces c-kit receptor-dependent mouse mast cell activation in vivo: evidence that signaling through the c-kit receptor can induce expression of cellular function. J Exp Med 175:245–255. doi:10.1084/jem.175.1.245
Reber L, Da Silva CA, Frossard N (2006) Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol 533:327–340. doi:10.1016/j.ejphar.2005.12.067
Möller C, Alfredsson J, Engström M et al (2005) Stem cell factor promotes mast cell survival via inactivation of FOXO3a-mediated transcriptional induction and MEK-regulated phosphorylation of the proapoptotic protein Bim. Blood 106:1330–1336. doi:10.1182/blood-2004-12-4792
Asai K, Kitaura J, Kawakami Y et al (2001) Regulation of mast cell survival by IgE. Immunity 14:791–800. doi:10.1016/S1074-7613(01)00157-1
Alfredsson J, Puthalakath H, Martin H, Strasser A, Nilsson G (2005) Proapoptotic Bcl-2 family member Bim is involved in the control of mast cell survival and is induced together with Bcl-xL upon IgE-receptor activation. Cell Death Differ 12:136–144. doi:10.1038/sj.cdd.4401537
Kalesnikoff J, Huber M, Lam V et al (2001) Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity 14:801–811. doi:10.1016/S1074-7613(01)00159-5
Kitaura J, Song J, Tsai M et al (2003) Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcepsilonRI. Proc Natl Acad Sci USA 100:12911–12916. doi:10.1073/pnas.1735525100
Hartmann K, Wagelie-Steffen AL, von Stebut E, Metcalfe DD (1997) Fas (CD95, APO-1) antigen expression and function in murine mast cells. J Immunol 159:4006–4014
Berent-Maoz B, Piliponsky AM, Daigle I, Simon HU, Levi-Schaffer F (2006) Human mast cells undergo TRAIL-induced apoptosis. J Immunol 176:2272–2278
Berent-Maoz B, Salemi S, Mankuta D, Simon HU, Levi-Schaffer F (2008) TRAIL mediated signaling in human mast cells: the influence of IgE-dependent activation. Allergy 63:333–340. doi:10.1111/j.1398-9995.2007.01598.x
Ekoff M, Kaufmann T, Engström M et al (2007) The BH3-only protein Puma plays an essential role in cytokine deprivation induced apoptosis of mast cells. Blood 110:3209–3217. doi:10.1182/blood-2007-02-073957
Simon D, Simon HU (2007) Eosinophilic disorders. J Allergy Clin Immunol 119:1291–1300. doi:10.1016/j.jaci.2007.02.010
Rothenberg ME, Hogan SP (2006) The eosinophil. Annu Rev Immunol 24:147–174. doi:10.1146/annurev.immunol.24.021605.090720
Sanderson CJ (1992) Interleukin-5, eosinophils, and disease. Blood 79:3101–3109
Owen WF, Rothenberg ME, Petersen J et al (1989) Interleukin 5 and phenotypically altered eosinophils in the blood of patients with the idiopathic hypereosinophilic syndrome. J Exp Med 170:343–348. doi:10.1084/jem.170.1.343
Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K (1997) Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol 158:3902–3908
Tumes DJ, Cormie J, Calvert MG et al (2007) Strain-dependent resistance to allergen-induced lung pathophysiology in mice correlates with rate of apoptosis of lung-derived eosinophils. J Leukoc Biol 81:1362–1373. doi:10.1189/jlb.0106046
Xu J, Jiang F, Nayeri F, Zetterström O (2007) Apoptotic eosinophils in sputum from asthmatic patients correlate negatively with levels of IL-5 and eotaxin. Respir Med 101:1447–1454. doi:10.1016/j.rmed.2007.01.026
Farahi N, Cowburn AS, Upton PD et al (2007) Eotaxin-1/CC chemokine ligand 11: A novel eosinophil survival factor secreted by human pulmonary artery endothelial cells. J Immunol 179:1264–1273
Conus S, Bruno A, Simon HU (2005) Leptin is an eosinophil survival factor. J Allergy Clin Immunol 116:1228–1234. doi:10.1016/j.jaci.2005.09.003
Bureau F, Seumois G, Jaspar F et al (2002) CD40 engagement enhances eosinophil survival through induction of cellular inhibitor of apoptosis protein 2 expression: possible involvement in allergic inflammation. J Allergy Clin Immunol 110:443–449. doi:10.1067/mai.2002.126781
Dibbert B, Daigle I, Braun D et al (1998) Role for Bcl-xL in delayed eosinophil apoptosis by granulocyte-macrophage colony-stimulating factor and interleukin-5. Blood 92:778–783
Segal M, Niazi S, Simons MP, Galati SA, Zangrilli JG (2007) Bid activation during induction of extrinsic and intrinsic apoptosis in eosinophils. Immunol Cell Biol 85:518–524. doi:10.1038/sj.icb.7100075
Dewson G, Cohen GM, Wardlaw AJ (2001) Interleukin-5 inhibits translocation of Bax to mitochondria, cytochrome c release, and activation of caspases in human eosinophils. Blood 98:2239–2247. doi:10.1182/blood.V98.7.2239
Vassina EM, Yousefi S, Simon D, Zwicky C, Conus S, Simon HU (2006) cIAP-2 and survivin contribute to cytokine-mediated delayed eosinophil apoptosis. Eur J Immunol 36:1975–1984. doi:10.1002/eji.200635943
Pazdrak K, Schreiber D, Forsythe P, Justement L, Alam R (1995) The signal transduction mechanism of IL-5 in eosinophils: the involvement of lyn tyrosine kinase and the ras-raf 1-MEK-MAP kinase pathway. J Exp Med 181:1827–1834. doi:10.1084/jem.181.5.1827
Yousefi S, Hoessli DC, Blaser K, Mills GB, Simon HU (1996) Requirement of Lyn and Syk tyrosine kinases for the prevention of apoptosis by cytokines in human eosinophils. J Exp Med 183:1407–1414. doi:10.1084/jem.183.4.1407
Pinho V, Souza DG, Barsante MM et al (2005) Phosphoinositide-3 kinases critically regulate the recruitment and survival of eosinophils in vivo: importance for the resolution of allergic inflammation. J Leukoc Biol 77:800–810. doi:10.1189/jlb.0704386
van der Bruggen T, Caldenhoven E, Kanters D et al (1995) Interleukin-5 signaling in human eosinophils involves JAK2 tyrosine kinase and STAT1α. Blood 85:1442–1448
Simon HU, Yousefi S, Dibbert B, Levi-Schaffer F, Blaser K (1997) Anti-apoptotic signals of granulocyte-macrophage colony-stimulating factor are transduced via Jak2 tyrosine kinase in eosinophils. Eur J Immunol 27:3536–3539. doi:10.1002/eji.1830271256
Fujihara S, Jaffray E, Farrow SN, Rossi AG, Haslett C, Hay RT (2005) Inhibition of NF-kappa B by a cell permeable form of I kappa B alpha induces apoptosis in eosinophils. Biochem Biophys Res Commun 326:632–637. doi:10.1016/j.bbrc.2004.11.090
Hasala H, Zhang X, Saarelainen S, Moilanen E, Kankaanranta H (2007) c-Jun N-terminal kinase mediates constitutive human eosinophil apoptosis. Pulm Pharmacol Ther 20:580–587. doi:10.1016/j.pupt.2006.06.004
Matsumoto K, Schleimer RP, Saito H, Iikura Y, Bochner BS (1995) Induction of apoptosis in human eosinophils by anti-Fas antibody treatment in vitro. Blood 86:1437–1443
Hebestreit H, Dibbert B, Balatti I et al (1998) Disruption of Fas receptor signaling by nitric oxide in eosinophils. J Exp Med 187:415–425. doi:10.1084/jem.187.3.415
Daigle I, Simon HU (2001) Alternative functions for TRAIL receptors in eosinophils and neutrophils. Swiss Med Wkly 131:231–237
Temkin V, Levi-Schaffer F (2001) Mechanisms of tumour necrosis factor alpha mediated eosinophil survival. Cytokine 15:20–26. doi:10.1006/cyto.2001.0890
Mahajan L, Madan T, Kamal N et al (2008) Recombinant surfactant protein-D selectively increases apoptosis in eosinophils of allergic asthmatics and enhances uptake of apoptotic eosinophils by macrophages. Int Immunol 20:993–1007. doi:10.1093/intimm/dxn058
Nutku E, Aizawa H, Hudson SA, Bochner BS (2003) Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 101:5014–5020. doi:10.1182/blood-2002-10-3058
Nutku-Bilir E, Hudson SA, Bochner BS (2008) Interleukin-5 priming of human eosinophils alters Siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol 38:121–124. doi:10.1165/rcmb.2007-0154OC
von Gunten S, Simon HU (2007) Autophagic-like cell death in neutrophils induced by autoantibodies. Autophagy 3:67–68
Bochner BS, Alvarez RA, Mehta P et al (2005) Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem 280:4307–4312. doi:10.1074/jbc.M412378200
von Gunten S, Vogel M, Schaub A et al (2007) Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol 119:1005–1011. doi:10.1016/j.jaci.2007.01.023
Zimmermann N, McBride ML, Yamada Y et al (2008) Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy 63:1156–1163. doi:10.1111/j.1398-9995.2008.01709.x
Meagher LC, Cousin JM, Seckl JR, Haslett C (1996) Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol 156:4422–4428
Woolley KL, Gibson PG, Carty K, Wilson AJ, Twaddell SH, Woolley MJ (1996) Eosinophil apoptosis and the resolution of airway inflammation in asthma. Am J Respir Crit Care Med 154:237–243
Yasui K, Hu B, Nakazawa T, Agematsu K, Komiyama A (1997) Theophylline accelerates human granulocyte apoptosis not via phosphodiesterase inhibition. J Clin Invest 100:1677–1684. doi:10.1172/JCI119692
Hallsworth MP, Giembycz MA, Barnes PJ, Lee TH (1996) Cyclic AMP-elevating agents prolong or inhibit eosinophil survival depending on prior exposure to GM-CSF. Br J Pharmacol 117:79–86
Shaw DE, Berry MA, Hargadon B et al (2007) Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest 132:1871–1875. doi:10.1378/chest.07-1047
Daigle I, Yousefi S, Colonna M, Green DR, Simon HU (2002) Death receptors bind SHP-1 and block cytokine-induced anti-apoptotic signalling in neutrophils. Nat Med 8:61–67. doi:10.1038/nm0102-61
Perianayagam MC, Balakrishnan VS, King AJ, Pereira BJ, Jaber BL (2002) C5a delays apoptosis of human neutrophils by a phosphatidylinositol 3-kinase-signaling pathway. Kidney Int 61:456–463. doi:10.1046/j.1523-1755.2002.00139.x
von Gunten S, Yousefi S, Seitz M et al (2005) Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood 106:1423–1431. doi:10.1182/blood-2004-10-4112
Kostylina G, Simon D, Fey MF, Yousefi S, Simon HU (2008) Neutrophil apoptosis mediated by nicotinic acid receptors (GPR109A). Cell Death Differ 15:134–142. doi:10.1038/sj.cdd.4402238
Fuchs TA, Abed U, Goosmann C et al (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176:231–241. doi:10.1083/jcb.200606027
Yousefi S, Gold JA, Andina N et al (2008) Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med 14:949–953. doi:10.1038/nm.1855
Walmsley SR, Print C, Farahi N et al (2005) Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NF-κB activity. J Exp Med 201:105–115. doi:10.1084/jem.20040624
Yousefi S, Green DR, Blaser K, Simon HU (1994) Protein-tyrosine phosphorylation regulates apoptosis in human eosinophils and neutrophils. Proc Natl Acad Sci USA 91:10868–10872. doi:10.1073/pnas.91.23.10868
Daigle I, Simon HU (2001) Critical role for caspases 3 and 8 in neutrophil but not eosinophil apoptosis. Int Arch Allergy Immunol 126:147–156. doi:10.1159/000049506
Altznauer F, Martinelli S, Yousefi S et al (2004) Inflammation-associated cell cycle-independent block of apoptosis by survivin in terminally differentiated neutrophils. J Exp Med 199:1343–1354. doi:10.1084/jem.20032033
Villunger A, Scott C, Bouillet P, Strasser A (2003) Essential role for the BH3-only protein Bim, but redundant roles for Bax, Bcl-2 and Bcl-w in the control of granulocyte survival. Blood 101:2393–2400. doi:10.1182/blood-2002-07-2132
Zheng X, Karsan A, Duronio V et al (2002) Interleukin-3, but not granulocyte-macrophage colony-stimulating factor and interleukin-5 inhibits apoptosis of human basophils through phosphatidylinositol 3-kinase: requirement of NF-κB-dependent and -independent pathways. Immunol 107:306–315. doi:10.1046/j.1365-2567.2002.01517.x
Matsumoto K, Maeda A, Bochner BS, Wakiguchi H, Saito H (2008) Induction of apoptosis in human basophils by anti-Fas antibody treatment in vitro. Int Arch Allergy Immunol 146(suppl. 1):40–46. doi:10.1159/000126060
Didichenko SA, Spiegl N, Brunner T, Dahinden CA (2008) IL-3 induces a Pim1-dependent anti-apoptotic pathway in primary human basophils. Blood. doi:10.1182/blood-2008-04-149419
Umetsu DT, DeKruyff RH (2006) The regulation of allergy and asthma. Immunol Rev 212:238–255. doi:10.1111/j.0105-2896.2006.00413.x
Akdis CA, Blaser K, Akdis M (2004) Apoptosis in tissue inflammation and allergic disease. Curr Opin Immunol 16:717–723. doi:10.1016/j.coi.2004.09.004
Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ (1993) Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75:229–240. doi:10.1016/0092-8674(93)80065-M
Vella AT, Dow S, Potter TA, Kappler J, Marrack P (1998) Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA 95:3810–3815. doi:10.1073/pnas.95.7.3810
Li XC, Demirci G, Ferrari-Lacraz S et al (2001) IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med 7:114–118. doi:10.1038/83253
Kirberg J, Berns A, von Boehmer H (1997) Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J Exp Med 186:1269–1275. doi:10.1084/jem.186.8.1269
Duke RC, Cohen JJ (1986) IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res 5:289–299
Hildeman DA, Zhu Y, Mitchell TC et al (2002) Activated T cell death in vivo mediated by pro-apoptotic Bcl-2 family member Bim. Immunity 16:759–767. doi:10.1016/S1074-7613(02)00322-9
Arnold R, Brenner D, Becker M, Frey CR, Krammer PH (2006) How T lymphocytes switch between life and death. Eur J Immunol 36:1654–1658. doi:10.1002/eji.200636197
Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK (1998) Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity 8:615–623. doi:10.1016/S1074-7613(00)80566-X
Zhang XR, Zhang LY, Devadas S, Li L, Keegan AD, Shi YF (2003) Reciprocal expression of TRAIL and CD95L in Th1 and Th2 cells: role of apoptosis in T helper subset differentiation. Cell Death Differ 10:203–210. doi:10.1038/sj.cdd.4401138
Akdis M, Trautmann A, Klunker S et al (2003) T helper (Th) 2 predominance in atopic disease is due to preferential apoptosis of circulating memory/effector Th1 cells. FASEB J 17:1026–1035. doi:10.1096/fj.02-1070com
Akkoc T, de Koning PJ, Rückert B, Barlan I, Akdis M, Akdis CA (2008) Increased activation-induced cell death of high IFN-γ-producing Th1 cells as a mechanism of Th2 predominance in atopic diseases. J Allergy Clin Immunol 121:652–658. doi:10.1016/j.jaci.2007.12.1171
Rieux-Laucat F, Le Deist F, Hivroz C et al (1995) Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 268:1347–1349. doi:10.1126/science.7539157
Fisher GH, Rosenberg FJ, Straus SE et al (1995) Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 81:935–946. doi:10.1016/0092-8674(95)90013-6
Devadas S, Das J, Liu C et al (2006) Granzyme B is critical for T cell receptor-induced cell death of type 2 helper T cells. Immunity 25:237–247. doi:10.1016/j.immuni.2006.06.011
Strasser A, Harris AW, Cory S (1991) Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 67:889–899. doi:10.1016/0092-8674(91)90362-3
Hoetzenecker W, Ecker R, Kopp T, Stuetz A, Stingl G, Elbe-Bürger A (2005) Pimecrolimus leads to an apoptosis-induced depletion of T cells but not Langerhans cells in patients with atopic dermatitis. J Allergy Clin Immunol 115:1276–1283. doi:10.1016/j.jaci.2005.02.011
Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ (2007) CD4+ CD25+ Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 8:1353–1362. doi:10.1038/ni1536
Maldonado-Lopez R, Maliszewski C, Urbain J, Moser M (2001) Cytokines regulate the capacity of CD8alpha(+) and CD8alpha(−) dendritic cells to prime Th1/Th2 cells in vivo. J Immunol 167:4345–4350
Harris NL, Ronchese F (1999) The role of B7 costimulation in T-cell immunity. Immunol Cell Biol 77:304–311. doi:10.1046/j.1440-1711.1999.00835.x
Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P (2000) Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instructions. J Immunol 164:4507–4512
van Rijt LS, Jung S, Kleinjan A et al (2005) In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 201:981–991. doi:10.1084/jem.20042311
Kleinjan A, Willart M, van Rijt LS et al (2006) An essential role for dendritic cells in human and experimental allergic rhinitis. J Allergy Clin Immunol 118:1117–1125. doi:10.1016/j.jaci.2006.05.030
Ingulli E, Mondino A, Khoruts A, Jenkins MK (1997) In vivo detection of dendritic cell antigen presentation to CD4+ T cells. J Exp Med 185:2133–2141. doi:10.1084/jem.185.12.2133
Park Y, Lee SW, Sung YC (2002) Cutting edge: CpG DNA inhibits dendritic cell apoptosis by up-regulating cellular inhibitor of apoptosis proteins through the phosphatidylinositide-3′-OH kinase pathway. J Immunol 168:5–8
Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P (1998) Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med 188:2175–2180. doi:10.1084/jem.188.11.2175
Wong BR, Josien R, Lee SY et al (1997) TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med 186:2075–2080. doi:10.1084/jem.186.12.2075
Hou WS, van Parijs L (2004) A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat Immunol 5:583–589. doi:10.1038/ni1071
Arques JL, Regoli M, Bertelli E, Nicoletti C (2008) Persistence of apoptosis-resistant T cell-activating dendritic cell promotes T helper type-2 response and IgE antibody production. Mol Immunol 45:2177–2186. doi:10.1016/j.molimm.2007.12.004
Schuller E, Oppel T, Bornhövd E, Wetzel S, Wollenberg A (2004) Tacrolimus ointment causes inflammatory dendritic epidermal cell depletion but not Langerhans cell apoptosis in patients with atopic dermatitis. J Allergy Clin Immunol 114:137–143. doi:10.1016/j.jaci.2004.03.021
Trautmann A, Schmid-Grendelmeier P, Krüger K et al (2002) T cells and eosinophils cooperate in the induction of bronchial epithelial apoptosis in asthma. J Allergy Clin Immunol 109:329–337. doi:10.1067/mai.2002.121460
Trautmann A, Akdis M, Kleeman D et al (2000) T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenic role in eczematous dermatitis. J Clin Invest 106:25–35. doi:10.1172/JCI9199
Acknowledgments
The laboratory of the authors is supported by the Swiss National Science Foundation (grant no. 310000–107526) and the OPO Foundation (Zurich).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simon, HU. Cell death in allergic diseases. Apoptosis 14, 439–446 (2009). https://doi.org/10.1007/s10495-008-0299-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0299-1