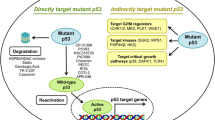
Abstract
p53 acts as a central mediator of the cellular response to stressful stimuli. The growth-suppressive function of p53 is lost with mutation and this occurs commonly in human cancer. In addition to suppressing cancer development and progression, wild-type p53 further confers chemo-sensitivity and radio-sensitivity upon tumor cells. Accumulated evidence over the last two decades that wild-type p53 activity is required for the efficacy of radiation and chemotherapy has led to considerable interest in development of strategies to restore normal p53 function in tumors with defective p53-dependent signaling. A number of promising discoveries, based on the knowledge of structural and functional basis of p53 mutation, p53 degradation by MDM2 and p53 family proteins, provide a foundation for future drug design. Here we review the role of p53 in enhancing the sensitivity from radiation and chemotherapy and discuss current progress on therapies targeting p53.

Similar content being viewed by others
References
Crawford LV, Lane DP (1977) An immune complex assay for SV40 T antigen. Biochem Biophys Res Commun 74:323–329. doi:10.1016/0006-291X(77)91411-5
Bassett EA, Wang W, Rastinejad F, El-Deiry WS (2008) Structural and functional basis for therapeutic modulation of p53 signaling. Clin Cancer Res 14:6376–6386. doi:10.1158/1078-0432.CCR-08-1526
El-Deiry WS (1998) Regulation of p53 downstream genes. Semin Cancer Biol 8:345–357. doi:10.1006/scbi.1998.0097
Wang W, Rastinejad F, El-Deiry WS (2003) Restoring p53-dependent tumor suppression. Cancer Biol Ther 2:S55–S63
Shangary S, Wang S (2008) Targeting the MDM2–p53 interaction for cancer therapy. Clin Cancer Res 14:5318–5324. doi:10.1158/1078-0432.CCR-07-5136
Dornan D, Wertz I, Shimizu H et al (2004) The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature 429:86–92. doi:10.1038/nature02514
Leng RP, Lin Y, Ma W et al (2003) Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell 112:779–791. doi:10.1016/S0092-8674(03)00193-4
Tang J, Qu LK, Zhang J et al (2006) Critical role for Daxx in regulating Mdm2. Nat Cell Biol 8:855–862. doi:10.1038/ncb1442
Li L, Deng B, Xing G et al (2007) PACT is a negative regulator of p53 and essential for cell growth and embryonic development. Proc Natl Acad Sci USA 104:7951–7956. doi:10.1073/pnas.0701916104
Yang W, El-Deiry WS (2007) CARPs are E3 ligases that target apical caspases and p53. Cancer Biol Ther 6:1676–1683. doi:10.1158/1535-7163.MCT-06-0557
Xu Y (2003) Regulation of p53 responses by post-translational modifications. Cell Death Differ 10:400–403. doi:10.1038/sj.cdd.4401182
Bode AM, Dong Z (2004) Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 4:793–805. doi:10.1038/nrc1455
Zhou J, Schmid T, Brune B (2004) HIF-1alpha and p53 as targets of NO in affecting cell proliferation, death and adaptation. Curr Mol Med 4:741–751. doi:10.2174/1566524043359926
Damalas A, Kahan S, Shtutman M, Ben-Ze’ev A, Oren M (2001) Deregulated? -catenin induces a p53- and ARF-dependent growth arrest and cooperates with Ras in transformation. EMBO J 20:4912–4922. doi:10.1093/emboj/20.17.4912
Esteller M, Tortola S, Toyota M et al (2000) Hypermethylation-associated inactivation of p14(ARF) is independent of p16(INK4a) methylation and p53 mutational status. Cancer Res 60:129–133
Feng L, Lin T, Uranishi H, Gu W, Xu Y (2005) Functional analysis of the roles of posttranslational modifications at the p53 C terminus in regulating p53 stability and activity. Mol Cell Biol 25:5389–5395. doi:10.1128/MCB.25.13.5389-5395.2005
Chan HM, La Thangue NB (2001) p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci 114:2363–2373
Gostissa M, Hengstermann A, Fogal V et al (1999) Activation of p53 by conjugation to the ubiquitin-like protein SUMO-1. EMBO J 18:6462–6471. doi:10.1093/emboj/18.22.6462
El-Deiry WS, Harper JW, O’Connor PM et al (1994) WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 54:1169–1174
Taylor WR, Stark GR (2001) Regulation of the G2/M transition by p53. Oncogene 20:1803–1815. doi:10.1038/sj.onc.1204252
Hermeking H, Benzinger A (2006) 14-3-3 proteins in cell cycle regulation. Semin Cancer Biol 16:183–192. doi:10.1016/j.semcancer.2006.03.002
Tan T, Chu G (2002) p53 binds and activates the xeroderma pigmentosum DDB2 gene in humans but not mice. Mol Cell Biol 22:3247–3254. doi:10.1128/MCB.22.10.3247-3254.2002
Tanaka H, Arakawa H, Yamaguchi T et al (2000) A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature 404:42–49. doi:10.1038/35003506
Takimoto R, MacLachlan TK, Dicker DT, Niitsu Y, Mori T, El-Deiry WS (2002) BRCA1 transcriptionally regulates damaged DNA binding protein (DDB2) in the DNA repair response following UV-irradiation. Cancer Biol Ther 1:177–186
Sax JK, El-Deiry WS (2003) p53 downstream targets and chemosensitivity. Cell Death Differ 10:413–417. doi:10.1038/sj.cdd.4401227
Fei P, Wang W, Kim SH et al (2004) Bnip3L is induced by p53 under hypoxia, and its knockdown promotes tumor growth. Cancer Cell 6:597–609. doi:10.1016/j.ccr.2004.10.012
Burns TF, El-Deiry WS (1999) The p53 pathway and apoptosis. J Cell Physiol 181:231–239. doi:10.1002/(SICI)1097-4652(199911)181:2<231::AID-JCP5>3.0.CO;2-L
Tanikawa C, Matsuda K, Fukuda S, Nakamura Y, Arakawa H (2003) p53RDL1 regulates p53-dependent apoptosis. Nat Cell Biol 5:216–223. doi:10.1038/ncb943
Oda E, Ohki R, Murasawa H et al (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053–1058. doi:10.1126/science.288.5468.1053
Wei MC, Zong WX, Cheng EHY et al (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727–730. doi:10.1126/science.1059108
Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L (2003) PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA 100:1931–1936. doi:10.1073/pnas.2627984100
Hutchinson F (1985) Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol 32:115–154. doi:10.1016/S0079-6603(08)60347-5
Fei P, El-Deiry WS (2003) P53 and radiation responses. Oncogene 22:5774–5783. doi:10.1038/sj.onc.1206677
Michelson R, Weinert T (1999) Sensor-less checkpoint activation? Nat Cell Biol 1:177–179. doi:10.1038/15614
Edwards RJ, Bentley NJ, Carr AMA (1999) Rad3-Rad26 complex responds to DNA damage independently of other checkpoint proteins. Nat Cell Biol 1:393–398. doi:10.1038/15623
Wang B, Matsuoka S, Carpenter PB, Elledge SJ (2002) 53BP1, a mediator of the DNA damage checkpoint. Science 298:1435–1438. doi:10.1126/science.1076182
Bakkenist CJ, Kastan MB (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499–506. doi:10.1038/nature01368
Lowe SW, Bodis S, McClatchey A et al (1994) p53 status and the efficacy of cancer therapy in vivo. Science 266:807–810. doi:10.1126/science.7973635
Schmitt CA, Lowe SW (2001) Bcl-2 mediates chemoresistance in matched pairs of primary Eμ-myc lymphomas in vivo. Blood Cells Mol Dis 27:206–216. doi:10.1006/bcmd.2000.0372
Komarova EA, Chernov MV, Franks R et al (1997) Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. EMBO J 16:1391–1400. doi:10.1093/emboj/16.6.1391
Fei P, Bernhard EJ, El-Deiry WS (2002) Tissue-specific induction of p53 targets in vivo. Cancer Res 62:7316–7327
El-Deiry WS (2003) The role of p53 in chemosensitivity and radiosensitivity. Oncogene 22:7486–7495. doi:10.1038/sj.onc.1206949
Sierra A, Castellsague X, Escobedo A et al (2000) Bcl-2 with loss of apoptosis allows accumulation of genetic alterations: a pathway to metastatic progression in human breast cancer. Int J Cancer 86:142–147. doi:10.1002/(SICI)1097-0215(20000320)89:2<142::AID-IJC7>3.0.CO;2-B
Kastan MB, Radin AI, Kuerbitz SJ et al (1991) Levels of p53 protein increase with maturation in human hematopoietic cells. Cancer Res 51:4279–4286
Komarov PG, Komarova EA, Kondratov RV et al (1999) A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285:1733–1737. doi:10.1126/science.285.5434.1733
Strom E, Sathe S, Komarov PG et al (2006) Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat Chem Biol 2:474–479. doi:10.1038/nchembio809
Shelling AN (1997) Role of p53 in drug resistance in ovarian cancer. Lancet 349:744–745. doi:10.1016/S0140-6736(05)60195-X
Hamada M, Fujiwara T, Hizuta A et al (1996) The p53 gene is a potent determinant of chemosensitivity and radiosensitivity in gastric and colorectal cancers. J Cancer Res Clin Oncol 122:360–365. doi:10.1007/BF01220804
Preudhomme C, Fenaux P (1997) The clinical significance of mutations of the P53 tumour suppressor gene in haematological malignancies. Br J Haematol 98:502–511. doi:10.1046/j.1365-2141.1997.2403057.x
Bergamaschi D, Gasco M, Hiller L et al (2003) p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell 3:387–402. doi:10.1016/S1535-6108(03)00079-5
Borresen AL, Andersen TI, Eyfjord JE et al (1995) Tp53 mutations and breast cancer prognosis: particularly poor survival rates for cases with mutations in the zinc-binding domains. Genes Chromosom Cancer 14:71–75. doi:10.1002/gcc.2870140113
Aas T, Børresen AL, Geisler S et al (1996) Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat Med 2:811–814. doi:10.1038/nm0796-811
Pritchard M, Watson AJM, Potten CS, Jackman AL, Hickman JA (1997) Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluorouracil evidence for the involvement of RNA perturbation. Proc Natl Acad Sci USA 94:1795–1799. doi:10.1073/pnas.94.5.1795
Bunz F, Hwang PM, Torrance C et al (1999) Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest 104:263–269. doi:10.1172/JCI6863
Blagosklonny MV, Fojo T (1999) Molecular effects of paclitaxel: myths and reality (a critical review). Int J Cancer 83:151–156. doi:10.1002/(SICI)1097-0215(19991008)83:2<151::AID-IJC1>3.0.CO;2-5
Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV, Fojo T (2000) p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat Cell Biol 2:709–717. doi:10.1038/35036335
Trielli MO, Andreassen PR, Lacroix FB, Margolis RL (1996) Differential taxol-dependent arrest of transformed and nontransformed cells in the G1 phase of the cell cycle, and specific-related mortality of transformed cells. J Cell Biol 135:689–700. doi:10.1083/jcb.135.3.689
Wang W, El-Deiry WS (2008) Restoration of p53 to limit tumor growth. Curr Opin Oncol 20:90–96
Wang S, El-Deiry WS (2004) The p53 pathway: targets for the development of novel cancer therapeutics. Cancer Treat Res 119:175–187. doi:10.1007/1-4020-7847-1_9
Zeimet AG, Marth C (2003) Why did p53 gene therapy fail in ovarian cancer? Lancet Oncol 4:415–422. doi:10.1016/S1470-2045(03)01139-2
Peng Z (2005) Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther 16:1016–1027. doi:10.1089/hum.2005.16.1016
Nemunaitis J, Swisher SG, Timmons T et al (2000) Adenovirus-mediated p53 gene transfer in sequence with cisplatin to tumors of patients with non-small-cell lung cancer. J Clin Oncol 18:609–622
Wilson JM (2005) Gendicine: the first commercial gene therapy product. Hum Gene Ther 16:1014–1015. doi:10.1089/hum.2005.16.1014
Bischoff JR, Kirn DH, Williams A et al (1996) An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274:373–376. doi:10.1126/science.274.5286.373
Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH (1997) ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med 3:639–645. doi:10.1038/nm0697-639
Rogulski KR, Freytag SO, Zhang K et al (2000) In vivo antitumor activity of ONYX-015 is influenced by p53 status and is augmented by radiotherapy. Cancer Res 60:1193–1196
Heise C, Lemmon M, Kirn D (2000) Efficacy with a replication-selective adenovirus plus cisplatin-based chemotherapy: dependence on sequencing but not p53 functional status or route of administration. Clin Cancer Res 6:4908–4914
Khuri FR, Nemunaitis J, Ganly I et al (2000) A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med 6:879–885. doi:10.1038/78638
Ganly I, Kirn D, Eckhardt G et al (2000) A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res 6:798–806
Brachmann RK, Yu K, Eby Y, Pavletich NP, Boeke JD (1998) Genetic selection of intragenic suppressor mutations that reverse the effect of common p53 cancer mutations. EMBO J 17:1847–1859. doi:10.1093/emboj/17.7.1847
Cui R, Widlund HR, Feige E et al (2007) Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell 128:853–864. doi:10.1016/j.cell.2006.12.045
Foster BA, Coffey HA, Morin MJ, Rastinejad F (1999) Pharmacological rescue of mutant p53 conformation and function. Science 286:2507–2510. doi:10.1126/science.286.5449.2507
Takimoto R, Wang W, Dicker DT, Rastinejad F, Lyssikatos J, El-Deiry WS (2002) The mutant p53-conformation modifying drug, CP-31398, can induce apoptosis of human cancer cells and can stabilize wild-type p53 protein. Cancer Biol Ther 1:47–55
Tang X, Zhu Y, Han L et al (2007) CP-31398 restores mutant p53 tumor suppressor function and inhibits UVB-induced skin carcinogenesis in mice. J Clin Invest 117:3753–3764. doi:10.1172/JCI32481
Rippin TM, Bykov VJ, Freund SM, Selivanova G, Wiman KG, Fersht AR (2002) Characterization of the p53-rescue drug CP-31398 in vitro and in living cells. Oncogene 21:2119–2129. doi:10.1038/sj.onc.1205362
Wang W, Takimoto R, Rastinejad F, El-Deiry WS (2003) Stabilization of p53 by CP-31398 inhibits ubiquitination without altering phosphorylation at serine 15 or 20 or MDM2 binding. Mol Cell Biol 23:2171–2181. doi:10.1128/MCB.23.6.2171-2181.2003
Luu Y, Bush J, Cheung KJ Jr, Li G (2002) The p53 stabilizing compound CP-31398 induces apoptosis by activating the intrinsic Bax/mitochondrial/caspase-9 pathway. Exp Cell Res 276:214–222. doi:10.1006/excr.2002.5526
Bykov VJ, Issaeva N, Shilov A et al (2002) Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med 8:282–288. doi:10.1038/nm0302-282
Magrini R, Russo D, Ottaggio L, Fronza G, Inga A, Menichini P (2008) PRIMA-1 synergizes with adriamycin to induce cell death in non-small cell lung cancer cells. J Cell Biochem 104:2363–2373. doi:10.1002/jcb.21794
Supiot S, Zhao H, Wiman K, Hill RP, Bristow RG (2008) PRIMA-1(met) radiosensitizes prostate cancer cells independent of their MTp53-status. Radiother Oncol 86:407–411. doi:10.1016/j.radonc.2008.01.001
Zache N, Lambert JM, Wiman KG, Bykov VJ (2008) PRIMA-1MET inhibits growth of mouse tumors carrying mutant p53. Cell Oncol 30:411–418
Bykov VJ, Zache N, Stridh H et al (2005) PRIMA-1(MET) synergizes with cisplatin to induce tumor cell apoptosis. Oncogene 24:3484–3491. doi:10.1038/sj.onc.1208419
Li Y, Mao Y, Brandt-Rauf PW, Williams AC, Fine RL (2005) Selective induction of apoptosis in mutant p53 premalignant and malignant cancer cells by PRIMA-1 through the c-Jun-NH2-kinase pathway. Mol Cancer Ther 4:901–909. doi:10.1158/1535-7163.MCT-04-0206
Rehman A, Chahal MS, Tang X, Bruce JE, Pommier Y, Daoud SS (2005) Proteomic identification of heat shock protein 90 as a candidate target for p53 mutation reactivation by PRIMA-1 in breast cancer cells. Breast Cancer Res 7:R765–R774. doi:10.1186/bcr1290
Friedler A, Hansson LO, Veprintsev DB et al (2002) A peptide that binds and stabilizes p53 core domain: chaperone strategy for rescue of oncogenic mutants. Proc Natl Acad Sci USA 99:937–942. doi:10.1073/pnas.241629998
Issaeva N, Friedler A, Bozko P, Wiman KG, Fersht AR, Selivanova G (2003) Rescue of mutants of the tumor suppressor p53 in cancer cells by a designed peptide. Proc Natl Acad Sci USA 100:13303–13307. doi:10.1073/pnas.1835733100
Hupp TR, Sparks A, Lane DP (1995) Small peptides activate the latent sequence-specific DNA binding function of p53. Cell 83:237–245. doi:10.1016/0092-8674(95)90165-5
Selivanova G, Ryabchenko L, Jansson E, Iotsova V, Wiman KG (1999) Reactivation of mutant p53 through interaction of a C-terminal peptide with the core domain. Mol Cell Biol 19:3395–3402
Wang W, El-Deiry WS (2004) Targeting p53 by PTD-mediated transduction. Trends Biotechnol 22:431–434. doi:10.1016/j.tibtech.2004.07.002
Kim AL, Raffo AJ, Brandt-Rauf PW et al (1999) Conformational and molecular basis for induction of apoptosis by a p53 C-terminal peptide in human cancer cells. J Biol Chem 274:34924–34931. doi:10.1074/jbc.274.49.34924
Snyder EL, Meade BR, Saenz CC, Dowdy SF (2004) Treatment of terminal peritoneal carcinomatosis by a transducible p53-activating peptide. PLoS Biol 2(2):E36
Peng Y, Li C, Chen L, Sebti S, Chen J (2003) Rescue of mutant p53 transcription function by ellipticine. Oncogene 22:4478–4487. doi:10.1038/sj.onc.1206777
Xu GW, Mawji IA, Macrae CJ et al (2008) A high-content chemical screen identifies ellipticine as a modulator of p53 nuclear localization. Apoptosis 13:413–422. doi:10.1007/s10495-007-0175-4
Sugikawa E, Hosoi T, Yazaki N, Gamanuma M, Nakanishi N, Ohashi M (1999) Mutant p53 mediated induction of cell cycle arrest and apoptosis at G1 phase by 9-hydroxyellipticine. Anticancer Res 19:3099–3108
Shi LM, Myers TG, Fan Y et al (1998) Mining the national cancer institute anticancer drug discovery database: cluster analysis of ellipticine analogs with p53-inverse and central nervous system-selective patterns of activity. Mol Pharmacol 53:241–251
Klein C, Vassilev LT (2004) Targeting the p53-MDM2 interaction to treat cancer. Br J Cancer 91:1415–1419
Vassilev LT, Vu BT, Graves B et al (2004) In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303:844–848. doi:10.1126/science.1092472
Gu L, Zhu N, Findley HW, Zhou M (2008) MDM2 antagonist nutlin-3 is a potent inducer of apoptosis in pediatric acute lymphoblastic leukemia cells with wild-type p53 and overexpression of MDM2. Leukemia 22:730–739. doi:10.1038/leu.2008.11
Laurie NA, Donovan SL, Shih CS et al (2006) Inactivation of the p53 pathway in retinoblastoma. Nature 444:61–66. doi:10.1038/nature05194
Coll-Mulet L, Iglesias-Serret D, Santidrian AF et al (2006) MDM2 antagonists activate p53 and synergize with genotoxic drugs in B-cell chronic lymphocytic leukemia cells. Blood 107:4109–4114. doi:10.1182/blood-2005-08-3273
Kojima K, Konopleva M, McQueen T, O’Brien S, Plunkett W, Andreeff M (2006) Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood 108:993–1000. doi:10.1182/blood-2005-12-5148
Barbieri E, Mehta P, Chen Z et al (2006) MDM2 inhibition sensitizes neuroblastoma to chemotherapy-induced apoptotic cell death. Mol Cancer Ther 5:2358–2365. doi:10.1158/1535-7163.MCT-06-0305
Supiot S, Hill RP, Bristow RG (2008) Nutlin-3 radiosensitizes hypoxic prostate cancer cells independent of p53. Mol Cancer Ther 7:993–999. doi:10.1158/1535-7163.MCT-07-0442
Cao C, Shinohara ET, Subhawong TK et al (2006) Radiosensitization of lung cancer by nutlin, an inhibitor of murine double minute 2. Mol Cancer Ther 5:411–417. doi:10.1158/1535-7163.MCT-05-0356
Secchiero P, Zerbinati C, di Iasio MG et al (2007) Synergistic cytotoxic activity of recombinant TRAIL plus the non-genotoxic activator of the p53 pathway nutlin-3 in acute myeloid leukemia cells. Curr Drug Metab 8:395–403. doi:10.2174/138920007780655432
Jiang M, Pabla N, Murphy RF et al (2007) Nutlin-3 protects kidney cells during cisplatin therapy by suppressing Bax/Bak activation. J Biol Chem 282:2636–2645. doi:10.1074/jbc.M606928200
Kranz D, Dobbelstein M (2006) Nongenotoxic p53 activation protects cells against S-phase-specific chemotherapy. Cancer Res 66:10274–10280. doi:10.1158/0008-5472.CAN-06-1527
Shen H, Moran DM, Maki CG (2008) Transient nutlin-3a treatment promotes endoreduplication and the generation of therapy-resistant tetraploid cells. Cancer Res 68:8260–8268. doi:10.1158/0008-5472.CAN-08-1901
Martins CP, Brown-Swigart L, Evan GI (2006) Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 127:1323–1334. doi:10.1016/j.cell.2006.12.007
Issaeva N, Bozko P, Enge M et al (2004) Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med 10:1321–1328. doi:10.1038/nm1146
Krajewski M, Ozdowy P, D’Silva L, Rothweiler U, Holak TA (2005) NMR indicates that the small molecule RITA does not block p53-MDM2 binding in vitro. Nat Med 11:1135–1136. doi:10.1038/nm1105-1135
Ding K, Lu Y, Nikolovska-Coleska Z et al (2006) Structure-based design of spiro-oxindoles as potent, specific small-molecule inhibitors of the MDM2–p53 interaction. J Med Chem 49:3432–3435. doi:10.1021/jm051122a
Shangary S, Qin D, McEachern D et al (2008) Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci USA 105:3933–3938. doi:10.1073/pnas.0708917105
Yang A, Kaghad M, Wang Y et al (1998) p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 2:305–316. doi:10.1016/S1097-2765(00)80275-0
Jost CA, Marin MC, Kaelin J (1997) p73 is a human p53-related protein that can induce apoptosis. Nature 389:191–194. doi:10.1038/38298
Flores ER, Sengupta S, Miller JB et al (2005) Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell 7:363–373. doi:10.1016/j.ccr.2005.02.019
Irwin MS, Kondo K, Marin MC, Cheng LS, Hahn WC, Kaelin J (2003) Chemosensitivity linked to p73 function. Cancer Cell 3:403–410. doi:10.1016/S1535-6108(03)00078-3
Bell HS, Dufes C, O’Prey J et al (2007) A p53-derived apoptotic peptide derepresses p73 to cause tumor regression in vivo. J Clin Invest 117:1008–1018. doi:10.1172/JCI28920
Bell HS, Ryan KM (2007) Targeting the p53 family for cancer therapy: ‘Big Brother’ joins the fight. Cell Cycle 6:1995–2000
Kravchenko JE, Ilyinskaya GV, Komarov PG et al (2008) Small-molecule RETRA suppresses mutant p53-bearing cancer cells through a p73-dependent salvage pathway. Proc Natl Acad Sci USA 105:6302–6307. doi:10.1073/pnas.0802091105
Wang W, Kim SH, El-Deiry WS (2006) Small-molecule modulators of p53 family signaling and antitumor effects in p53-deficient human colon tumor xenografts. Proc Natl Acad Sci USA 103:11003–11008. doi:10.1073/pnas.0604507103
Wang W, Ho WC, Dicker DT et al (2005) Acridine derivatives activate p53 and induce tumor cell death through Bax. Cancer Biol Ther 4:893–898. doi:10.1158/1535-7163.MCT-05-0048
Graat HC, Carette JE, Schagen FH et al (2007) Enhanced tumor cell kill by combined treatment with a small-molecule antagonist of mouse double minute 2 and adenoviruses encoding p53. Mol Cancer Ther 6:1552–1561. doi:10.1158/1535-7163.MCT-06-0631
Galluzzi L, Morselli E, Kepp O, Tajeddine N, Kroemer G (2008) Targeting p53 to mitochondria for cancer therapy. Cell Cycle 7:1949–1955
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, C., El-Deiry, W.S. Targeting p53 for enhanced radio- and chemo-sensitivity. Apoptosis 14, 597–606 (2009). https://doi.org/10.1007/s10495-009-0330-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-009-0330-1