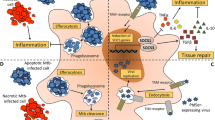
Abstract
Phagocytosis of dying cells is a complex and dynamic process coordinated by the interaction of many surface molecules, adaptors, and chemotactic molecules, and it is controlled at multiple levels. This well regulated clearance process is of utmost importance for the development and homeostasis of organisms because defective or inefficient phagocytosis may contribute to human pathologies. In this review we discuss recent advances in the knowledge of the molecular interactions involved in recognition and clearance of apoptotic cells and how derangement of these processes can contribute to the pathogenesis of chronic airway diseases such as chronic obstructive pulmonary disease, cystic fibrosis and asthma. We will briefly consider how different types of macrophages are implicated in chronic airway diseases. Finally, we will address possible therapeutic strategies, such as the use of macrolide antibiotics and statins, for modulating apoptotic cell clearance.

Similar content being viewed by others
References
Barnes PJ (2009) The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 41:631–638
Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG (2006) Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res 7:53
Henson PM, Tuder RM (2008) Apoptosis in the lung: induction, clearance and detection. Am J Physiol 294:601–611
Tuder RM, Petrache I, Elias JA, Voelkel NF, Henson PM (2003) Apoptosis and emphysema: the missing link. Am J Respir Cell Mol Biol 28:551–554
Imai K, Mercer BA, Schulman LL, Sonett JR, D’Armiento JM (2005) Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J 25:250–258
Makris D, Vrekoussis T, Izoldi M et al (2009) Increased apoptosis of neutrophils in induced sputum of COPD patients. Respir Med 103:1130–1135
Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M (2000) Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest 117:684–694
Uhal BD, Joshi I, Hughes WF, Ramos C, Pardo A, Selman M (1998) Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol 275:L1192–L1199
Hodge S, Hodge G, Holmes M, Reynolds PN (2005) Increased peripheral blood T-cell apoptosis and decreased Bcl-2 in chronic obstructive pulmonary disease. Immunol Cell Biol 83:160–166
Kasahara Y, Tuder RM, Cool CD, Voelkel NF (2000) Expression of 15-lipoxygenase and evidence for apoptosis in the lungs from patients with COPD. Chest 117:260S
Calabrese F, Giacometti C, Beghe B, et al (2005) Marked alveolar apoptosis/proliferation imbalance in end-stage emphysema. Respir Res 6:14–26
Yokohori N, Aoshiba K, Nagai A (2004) Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest 125:626–632
Rangasamy T, Misra V, Zhen L, Tankersley CG, Tuder RM, Biswal S (2009) Cigarette smoke-induced emphysema in A/J mice is associated with pulmonary oxidative stress, apoptosis of lung cells, and global alterations in gene expression. Am J Physiol 296:L888–L900
Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M (2003) Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol 81:289–296
Nakamura Y, Romberger DJ, Tate L et al (1995) Cigarette smoke inhibits lung fibroblast proliferation and chemotaxis. Am J Respir Crit Care Med 151:1497–1503
Rennard SI, Togo S, Holz O (2006) Cigarette smoke inhibits alveolar repair: a mechanism for the development of emphysema. Proc Am Thorac Soc 3:703–708
Hoshino S, Yoshida M, Inoue K et al (2005) Cigarette smoke extract induces endothelial cell injury via JNK pathway. Biochem Biophys Res Commun 329:58–63
Hodge S, Hodge G, Ahern J, Jersmann H, Holmes M, Reynolds PN (2007) Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 37:748–755
Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM (1999) The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol 9:1441–1447
Dini L, Vergallo C (2009) Environmental factors affecting phagocytosis of dying cells: smoking and static magnetic fields. In: Krysko DV, Vandenabeele P (eds) Phagocytosis of dying cells. Springer, Dordrecht, pp 409–438
Richens TR, Linderman DJ, Horstmann SA et al (2009) Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA. Am J Respir Crit Care Med 179:1011–1021
D’Hulst AI, Bracke KR, Maes T et al (2006) Role of tumour necrosis factor-alpha receptor p75 in cigarette smoke-induced pulmonary inflammation and emphysema. Eur Respir J 28:102–112
Churg A, Dai J, Tai H, Xie C, Wright JL (2002) Tumor necrosis factor-alpha is central to acute cigarette smoke-induced inflammation and connective tissue breakdown. Am J Respir Crit Care Med 166:849–854
Keatings VM, Collins PD, Scott DM, Barnes PJ (1996) Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 153:530–534
McPhillips K, Janssen WJ, Ghosh M et al (2007) TNF-alpha inhibits macrophage clearance of apoptotic cells via cytosolic phospholipase A2 and oxidant-dependent mechanisms. J Immunol 178:8117–8126
Borges VM, Vandivier RW, McPhillips KA et al (2009) TNFalpha inhibits apoptotic cell clearance in the lung, exacerbating acute inflammation. Am J Physiol 297:586–595
Kirkham PA, Spooner G, Rahman I, Rossi AG (2004) Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem Biophys Res Commun 318:32–37
Krysko DV, D’Herde K, Vandenabeele P (2006) Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis 11:1709–1726
Krysko DV, Vandenabeele P (2008) From regulation of dying cell engulfment to development of anti-cancer therapy. Cell Death Differ 15:29–38
Kazeros A, Harvey BG, Carolan BJ, Vanni H, Krause A, Crystal RG (2008) Overexpression of apoptotic cell removal receptor MERTK in alveolar macrophages of cigarette smokers. Am J Respir Cell Mol Biol 39:747–757
Pastva AM, Wright JR, Williams KL (2007) Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc 4:252–257
Wright JR (2005) Immunoregulatory functions of surfactant proteins. Nat Rev 5:58–68
Ohya M, Nishitani C, Sano H et al (2006) Human pulmonary surfactant protein D binds the extracellular domains of Toll-like receptors 2 and 4 through the carbohydrate recognition domain by a mechanism different from its binding to phosphatidylinositol and lipopolysaccharide. Biochemistry 45:8657–8664
Yamada C, Sano H, Shimizu T et al (2006) Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J Biol Chem 281:21771–21780
Vandivier RW, Ogden CA, Fadok VA et al (2002) Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol 169:3978–3986
Kishore U, Greenhough TJ, Waters P et al (2006) Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol 43:1293–1315
Hodge S, Hodge G, Jersmann H et al (2008) Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178:139–148
Ohlmeier S, Vuolanto M, Toljamo T et al (2008) Proteomics of human lung tissue identifies surfactant protein A as a marker of chronic obstructive pulmonary disease. J Proteome Res 7:5125–5132
Vlachaki EM, Koutsopoulos AV, Tzanakis N et al (2010) Altered surfactant protein-a (Sp-a) expression in type Ii pneumocytes in copd. Chest 137:37–45
Honda Y, Takahashi H, Kuroki Y, Akino T, Abe S (1996) Decreased contents of surfactant proteins A and D in BAL fluids of healthy smokers. Chest 109:1006–1009
Schagat TL, Wofford JA, Wright JR (2001) Surfactant protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J Immunol 166:2727–2733
Reidy MF, Wright JR (2003) Surfactant protein A enhances apoptotic cell uptake and TGF-beta1 release by inflammatory alveolar macrophages. Am J Physiol 285:L854–L861
Janssen WJ, McPhillips KA, Dickinson MG et al (2008) Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha. Am J Respir Crit Care Med 178:158–167
Hodge S, Matthews G, Dean MM et al (2010) Therapeutic role for mannose-binding lectin in cigarette smoke-induced lung inflammation? Evidence from a murine model. Am J Respir Cell Mol Biol 42:235–242
Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA (2005) Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol 174:3220–3226
Feghali-Bostwick CA, Gadgil AS, Otterbein LE et al (2008) Autoantibodies in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 177:156–163
Lee SH, Goswami S, Grudo A et al (2007) Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med 13:567–569
Gadgil A, Zhu X, Sciurba FC, Duncan SR (2006) Altered T-cell phenotypes in chronic obstructive pulmonary disease. Proc Am Thorac Soc 3:487–488
van der Strate BW, Postma DS, Brandsma CA et al (2006) Cigarette smoke-induced emphysema: A role for the B cell? Am J Respir Crit Care Med 173:751–758
Cosio MG, Saetta M, Agusti A (2009) Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med 360:2445–2454
Eggleton P, Haigh R, Winyard PG (2008) Consequence of neo-antigenicity of the ‘altered self’. Rheumatology 47:567–571
Agusti A, MacNee W, Donaldson K, Cosio M (2003) Hypothesis: does COPD have an autoimmune component? Thorax 58:832–834
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23:549–555
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev 5:953–964
Mantovani A, Sica A, Locati M (2005) Macrophage polarization comes of age. Immunity 23:344–346
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686
Gratchev A, Schledzewski K, Guillot P, Goerdt S (2001) Alternatively activated antigen-presenting cells: molecular repertoire, immune regulation, and healing. Skin Pharmacol Appl Skin Physiol 14:272–279
Shaykhiev R, Krause A, Salit J et al (2009) Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. J Immunol 183:2867–2883
Reynolds PN, Hodge SJ (2009) Chapter 14. The impact of defective clearance of apoptotic cells in the pathogenesis of chronic lung diseases: chronic obstructive pulmonary disease, asthma and cystic fibrosis. In: Krysko DV, Vandenabeele P (eds) Phagocytosis of dying cells from molecular mechanisms to human diseases. Springer, Dordrecht, pp 393–407
Gaschler GJ, Zavitz CC, Bauer CM et al (2008) Cigarette smoke exposure attenuates cytokine production by mouse alveolar macrophages. Am J Respir Cell Mol Biol 38:218–226
Laan M, Bozinovski S, Anderson GP (2004) Cigarette smoke inhibits lipopolysaccharide-induced production of inflammatory cytokines by suppressing the activation of activator protein-1 in bronchial epithelial cells. J Immunol 173:4164–4170
Sopori M (2002) Effects of cigarette smoke on the immune system. Nat Rev 2:372–377
Li L, Hamilton RF Jr, Holian A (1999) Effect of acrolein on human alveolar macrophage NF-kappaB activity. Am J Physiol 277:L550–L557
Hagiwara E, Takahashi KI, Okubo T et al (2001) Cigarette smoking depletes cells spontaneously secreting Th(1) cytokines in the human airway. Cytokine 14:121–126
Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 133:775–787
Rowe SM, Miller S, Sorscher EJ (2005) Cystic fibrosis. N Engl J Med 352:1992–2001
Vandivier RW, Fadok VA, Ogden CA et al (2002) Impaired clearance of apoptotic cells from cystic fibrosis airways. Chest 121:89S
Maiuri L, Raia V, De Marco G et al (1997) DNA fragmentation is a feature of cystic fibrosis epithelial cells: a disease with inappropriate apoptosis? FEBS Lett 408:225–231
Di A, Brown ME, Deriy LV et al (2006) CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol 8:933–944
Vandivier RW, Richens TR, Horstmann SA et al (2009) Dysfunctional cystic fibrosis transmembrane conductance regulator inhibits phagocytosis of apoptotic cells with proinflammatory consequences. Am J Physiol 297:677–686
Kreiselmeier NE, Kraynack NC, Corey DA, Kelley TJ (2003) Statin-mediated correction of STAT1 signaling and inducible nitric oxide synthase expression in cystic fibrosis epithelial cells. Am J Physiol 285:L1286–L1295
Elkin S, Geddes D (2003) Pseudomonal infection in cystic fibrosis: the battle continues. Expert Rev Anti Infect Ther 1:609–618
Bianchi SM, Prince LR, McPhillips K et al (2008) Impairment of apoptotic cell engulfment by pyocyanin, a toxic metabolite of Pseudomonas aeruginosa. Am J Respir Crit Care Med 177:35–43
Peter C, Wesselborg S, Lauber K (2009) Role of attraction and danger signals in the uptake of apoptotic and necrotic cells and its immunological outcome. In: Krysko DV, Vandenabeele P (eds) Phagocytosis of dying cells: from molecular mechanisms to human disease. Springer, Dordrecht, pp 63–101
Peter C, Wesselborg S, Herrmann M, Lauber K (2010) Dangerous attraction: phagocyte recruitment and danger signals of apoptotic and necrotic cells. Apoptosis, Feb 6 [Epub ahead of print]
Rowe SM, Jackson PL, Liu G et al (2008) Potential role of high-mobility group box 1 in cystic fibrosis airway disease. Am J Respir Crit Care Med 178:822–831
Liu G, Wang J, Park YJ et al (2008) High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J Immunol 181:4240–4246
del Fresno C, Garcia-Rio F, Gomez-Pina V et al (2009) Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J Immunol 182:6494–6507
Claeys S, Van Hoecke H, Holtappels G et al (2005) Nasal polyps in patients with and without cystic fibrosis: a differentiation by innate markers and inflammatory mediators. Clin Exp Allergy 35:467–472
Van Zele T, Claeys S, Gevaert P et al (2006) Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 61:1280–1289
Krysko O, Van Zele T, Claeys S, Bachert C (2009) Comment on “potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients”. J Immunol 183:4831; author reply 4831–4832
Van Hove CL, Maes T, Joos GF, Tournoy KG (2008) Chronic inflammation in asthma: a contest of persistence vs resolution. Allergy 63:1095–1109
Busse WW, Lemanske RF Jr (2001) Asthma. N Engl J Med 344:350–362
Solarewicz-Madejek K, Basinski TM, Crameri R et al (2009) T cells and eosinophils in bronchial smooth muscle cell death in asthma. Clin Exp Allergy 39:845–855
Walsh GM (2008) Defective apoptotic cell clearance in asthma and COPD—a new drug target for statins? Trends Pharmacol Sci 29:6–11
Duncan CJ, Lawrie A, Blaylock MG, Douglas JG, Walsh GM (2003) Reduced eosinophil apoptosis in induced sputum correlates with asthma severity. Eur Respir J 22:484–490
Woolley KL, Gibson PG, Carty K, Wilson AJ, Twaddell SH, Woolley MJ (1996) Eosinophil apoptosis and the resolution of airway inflammation in asthma. Am J Respir Crit Care Med 154:237–243
Huynh ML, Malcolm KC, Kotaru C et al (2005) Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med 172:972–979
Fitzpatrick AM, Holguin F, Teague WG, Brown LA (2008) Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol 121:1372–1378, 1378 e1371–1373
Alexis NE, Soukup J, Nierkens S, Becker S (2001) Association between airway hyperreactivity and bronchial macrophage dysfunction in individuals with mild asthma. Am J Physiol 280:L369–L375
Hodge S, Hodge G, Brozyna S, Jersmann H, Holmes M, Reynolds PN (2006) Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur Respir J 28:486–495
Allavena P, Chieppa M, Monti P, Piemonti L (2004) From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor. Crit Rev Immunol 24:179–192
Kobayashi H, Takeda H, Sakayori S et al (1995) Study on azithromycin in treatment of diffuse panbronchiolitis. Kansenshogaku Zasshi 69:711–722
Baumann U, King M, App EM et al (2004) Long term azithromycin therapy in cystic fibrosis patients: a study on drug levels and sputum properties. Can Respir J 11:151–155
Nakaya M, Tanaka M, Okabe Y, Hanayama R, Nagata S (2006) Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J Biol Chem 281:8836–8842
Eberlein M, Heusinger-Ribeiro J, Goppelt-Struebe M (2001) Rho-dependent inhibition of the induction of connective tissue growth factor (CTGF) by HMG CoA reductase inhibitors (statins). Br J Pharmacol 133:1172–1180
Morimoto K, Janssen WJ, Fessler MB et al (2006) Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol 176:7657–7665
McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY (2004) A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J Immunol 172:2903–2908
Menzies D, Nair A, Meldrum KT, Fleming D, Barnes M, Lipworth BJ (2007) Simvastatin does not exhibit therapeutic anti-inflammatory effects in asthma. J Allergy Clin Immunol 119:328–335
Benati D, Ferro M, Savino MT et al (2009) Opposite effects of simvastatin on the bactericidal and inflammatory response of macrophages to opsonized S. aureus. J Leukoc Biol 87:433–442
Maderna P, Godson C (2003) Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim Biophys Acta 1639:141–151
Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101:890–898
Golpon HA, Fadok VA, Taraseviciene-Stewart L et al (2004) Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J 18:1716–1718
Acknowledgements
This work was supported by the Fund for Scientific Research Flanders (FWO-Vlaanderen) (3G064210 to C.B., O.K. and 3G072810 to D.V.K.), by the Interuniversity Attraction Poles Programme (IUAP)—Belgian state—Belgian Science Policy (P6/35 to C.B.) and “krediet aan navorsers” from Fund for Scientific Research Flanders (FWO-Vlaanderen, 31507110 to D.V.K.). D.V.K. is a postdoctoral research fellow of the Fund for Scientific Research Flanders (FWO-Vlaanderen), Belgium. We thank Dr. A. Bredan for proofreading the manuscript and W. Drijvers for the art work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Olga Krysko and Dmitri V. Krysko contributed equally to this work.
Rights and permissions
About this article
Cite this article
Krysko, O., Vandenabeele, P., Krysko, D.V. et al. Impairment of phagocytosis of apoptotic cells and its role in chronic airway diseases. Apoptosis 15, 1137–1146 (2010). https://doi.org/10.1007/s10495-010-0504-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-010-0504-x