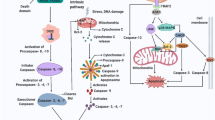
Abstract
Although several features of apoptosis and autophagy have been reported in the larval organs of Lepidoptera during metamorphosis, solid experimental evidence for autophagy is still lacking. Moreover, the role of the two processes and the nature of their relationship are still cryptic. In this study, we perform a cellular, biochemical and molecular analysis of the degeneration process that occurs in the larval midgut of Bombyx mori during larval–adult transformation, with the aim to analyze autophagy and apoptosis in cells that die under physiological conditions. We demonstrate that larval midgut degradation is due to the concerted action of the two mechanisms, which occur at different times and have different functions. Autophagy is activated from the wandering stage and reaches a high level of activity during the spinning and prepupal stages, as demonstrated by specific autophagic markers. Our data show that the process of autophagy can recycle molecules from the degenerating cells and supply nutrients to the animal during the non-feeding period. Apoptosis intervenes later. In fact, although genes encoding caspases are transcribed at the end of the larval period, the activity of these proteases is not appreciable until the second day of spinning and apoptotic features are observable from prepupal phase. The abundance of apoptotic features during the pupal phase, when the majority of the cells die, indicates that apoptosis is actually responsible for cell death and for the disappearance of larval midgut cells.












Similar content being viewed by others
References
Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH et al (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16:3–11
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV et al (2011) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. doi:10.1038/cdd.2011.96
Ulukaya E, Acilan C, Yilmaz Y (2011) Apoptosis: why and how does it occur in biology? Cell Biochem Funct. doi:10.1002/cbf.1774
He CC, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Berry DL, Baehrecke EH (2007) Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell 131:1137–1148
Velentzas AD, Nezis IP, Stravopodis DJ, Papassideri IS, Margaritis LH (2007) Apoptosis and autophagy function cooperatively for the efficacious execution of programmed nurse cell death during Drosophila virilis oogenesis. Autophagy 3:130–132
Denton D, Shravage B, Simin R, Mills K, Berry DL, Baehrecke EH et al (2009) Autophagy, not apoptosis, is essential for midgut cell death in Drosophila. Curr Biol 19:1741–1746
Nezis IP, Shravage BV, Sagona AP, Lamark T, Bjorkoy G, Johansen T et al (2010) Autophagic degradation of dBruce controls DNA fragmentation in nurse cells during late Drosophila melanogaster oogenesis. J Cell Biol 190:523–531
Shen HM, Codogno P (2011) Autophagic cell death: Loch Ness monster or endangered species? Autophagy 7:457–465
Melendez A, Neufeld TP (2008) The cell biology of autophagy in metazoans: a developing story. Development 135:2347–2360
Tettamanti G, Grimaldi A, Casartelli M, Ambrosetti E, Ponti B, Congiu T et al (2007) Programmed cell death and stem cell differentiation are responsible for midgut replacement in Heliothis virescens during prepupal instar. Cell Tissue Res 330:345–359
Tettamanti G, Grimaldi A, Pennacchio F, de Eguileor M (2007) Lepidopteran larval midgut during prepupal instar: digestion or self-digestion? Autophagy 3:630–631
Parthasarathy R, Palli SR (2007) Developmental and hormonal regulation of midgut remodeling in a lepidopteran insect, Heliothis virescens. Mech Dev 124:23–34
Malagoli D, Abdalla FC, Cao Y, Feng QL, Fujisaki K, Gregorc A et al (2010) Autophagy and its physiological relevance in arthropods: current knowledge and perspectives. Autophagy 6:575–588
Komuves LG, Sass M, Kovacs J (1985) Autophagocytosis in the larval midgut cells of Pieris brassicae during metamorphosis. Cell Tissue Res 240:215–221
Uwo MF, Ui-Tei K, Park P, Takeda M (2002) Replacement of midgut epithelium in the greater wax moth, Galleria mellonela, during larval–pupal moult. Cell Tissue Res 308:319–331
Dai JD, Gilbert LI (1997) Programmed cell death of the prothoracic glands of Manduca sexta during pupal-adult metamorphosis. Insect Biochem Mol Biol 27:69–78
Sumithra P, Britto CP, Krishnan M (2010) Modes of cell death in the pupal perivisceral fat body tissue of the silkworm Bombyx mori L. Cell Tissue Res 339:349–358
Dai JD, Gilbert LI (1999) An in vitro analysis of ecdysteroid-elicited cell death in the prothoracic gland of Manduca sexta. Cell Tissue Res 297:319–327
Muller F, Adori C, Sass M (2004) Autophagic and apoptotic features during programmed cell death in the fat body of the tobacco hornworm (Manduca sexta). Eur J Cell Biol 83:67–78
Lockshin RA, Zakeri Z (2004) Apoptosis, autophagy, and more. Int J Biochem Cell Biol 36:2405–2419
Silva-Zacarin EC, Tomaino GA, Brocheto-Braga MR, Taboga SR, De Moraes RL (2007) Programmed cell death in the larval salivary glands of Apis mellifera (Hymenoptera, Apidae). J Biosci 32:309–328
Facey COB, Lockshin RA (2010) The execution phase of autophagy associated PCD during insect metamorphosis. Apoptosis 15:639–652
Misch DW (1965) Alteration in subcellular structure of metamorphosing insect intestinal cells. Am Zool 5:699
Matsuura S, Tashiro Y (1976) Cup-shaped mitochondria in the posterior silk gland of Bombyx mori in the prepupal stadium. Cell Struct Funct 1:137–145
Lockshin R, Beaulaton J (1979) Programmed cell death. Electrophysiological and ultrastructural correlations in metamorphosing muscles of lepidopteran insects. Tissue Cell 11:803–819
Beaulaton J, Lockshin R (1977) Ultrastructural study of the normal degeneration of the intersegmental muscles of Anthereae polyphemus and Manduca sexta (Insecta, Lepidoptera) with particular reference of cellular autophagy. J Morphol 154:39–58
de Sousa MEC, Wanderley-Teixeira V, Teixeira AAC, de Siqueira HAA, Santos FAB, Alves LC (2009) Ultrastructure of the Alabama argillacea (Hubner) (Lepidoptera: Noctuidae) midgut. Micron 40:743–749
Xia Q, Wang J, Zhou Z, Li R, Fan W, Cheng D et al (2008) The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem Mol Biol 38:1036–1045
Tettamanti G, Cao Y, Feng C, Grimaldi A, de Eguileor M (2011) Autophagy in Lepidoptera: more than old wine in new bottle. Invert Surv J 8:5–14
Zhang X, Hu ZY, Li WF, Li QR, Deng XJ, Yang WY et al (2009) Systematic cloning and analysis of autophagy-related genes from the silkworm Bombyx mori. BMC Mol Biol 10:50
Li QR, Deng XJ, Yang WY, Huang ZJ, Tettamanti G, Cao Y et al (2010) Autophagy, apoptosis, and ecdysis-related gene expression in the silk gland of the silkworm (Bombyx mori) during metamorphosis. Can J Zool 88:1169–1178
Vilaplana L, Pascual N, Perera N, Belles X (2007) Molecular characterization of an inhibitor of apoptosis in the Egyptian armyworm, Spodoptera littoralis, and midgut cell death during metamorphosis. Insect Biochem Mol Biol 37:1241–1248
Goncu E, Parlak O (2008) Some autophagic and apoptotic features of programmed cell death in the anterior silk glands of the silkworm, Bombyx mori. Autophagy 4:1069–1072
Mpakou VE, Nezis IP, Stravopodis DJ, Margaritis LH, Papassideri IS (2006) Programmed cell death of the ovarian nurse cells during oogenesis of the silkmoth Bombyx mori. Dev Growth Differ 48:419–428
Hoffman KL, Weeks JC (2001) Role of caspases and mitochondria in the steroid-induced programmed cell death of a motoneuron during metamorphosis. Dev Biol 229:517–536
Kinch G, Hoffman KL, Rodrigues EM, Zee MC, Weeks JC (2003) Steroid-triggered programmed cell death of a motoneuron is autophagic and involves structural changes in mitochondria. J Comp Neurol 457:384–403
Mpakou VE, Nezis IP, Stravopodis DJ, Margaritis LH, Papassideri IS (2008) Different modes of programmed cell death during oogenesis of the silkmoth Bombyx mori. Autophagy 4:97–100
Zhong Y, Imanishi S, Kawasaki H (2005) Ecdysone responsiveness of several cell lines derived from Bombyx mori. J Insect Biotech Sericol 74:117–123
Hakim RS, Baldwin K, Smagghe G (2010) Regulation of midgut growth, development, and metamorphosis. Annu Rev Entomol 55:593–608
Truman JW, Riddiford LM (2002) Endocrine insights into the evolution of metamorphosis in insects. Annu Rev Entomol 47:467–500
Wu Y, Parthasarathy R, Bai H, Palli SR (2006) Mechanisms of midgut remodeling: juvenile hormone analog methoprene blocks midgut metamorphosis by modulating ecdysone action. Mech Dev 123:530–547
Tettamanti G, Grimaldi A, Pennacchio F, de Eguileor M (2008) Toxoneuron nigriceps parasitization delays midgut replacement in fifth-instar Heliothis virescens larvae. Cell Tissue Res 332:371–379
Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS et al (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4:151–175
Galluzzi L, Aaronson SA, Abrams J, Alnemri ES, Andrews DW, Baehrecke EH et al (2009) Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ 16:1093–1107
Cappellozza L, Cappellozza S, Saviane A, Sbrenna G (2005) Artificial diet rearing system for the silkworm Bombyx mori (Lepidoptera: Bombycidae): effect of vitamin C deprivation on larval growth and cocoon production. Appl Entomol Zool 40:405–412
Kiguchi K, Agui N (1981) Ecdysteroid levels and developmental events during larval moulting in the silkworm, Bombyx mori. J Insect Physiol 27:805–812
Yla-Anttila P, Vihinen H, Jokitalo E, Eskelinen EL (2009) Monitoring autophagy by electron microscopy in mammalian cells. Methods Enzymol 452:143–164
Tettamanti G, Malagoli D (2008) In vitro methods to monitor autophagy in Lepidoptera. Method Enzymol 451:685–709
Dartsch DC, Schaefer A, Boldt S, Kolch W, Marquardt H (2002) Comparison of anthracycline-induced death of human leukemia cells: programmed cell death versus necrosis. Apoptosis 7:537–548
Hu C, Zhang XA, Teng YB, Hu HX, Li WF (2010) Structure of autophagy-related protein Atg8 from the silkworm Bombyx mori. Acta Crystallogr F 66:787–790
Sambrook J, Fritsch EF, Maniatis T (1989) Detection and analysis of proteins expressed from cloned genes. In: Molecular cloning—a laboratory manual. Cold Spring Harbor Laboratory Press, New York, pp 18.11–18.88
Welinder C, Ekblad L (2011) Coomassie staining as loading control in Western blot analysis. J Proteome Res 10:1416–1419
Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Martinez-Augustin O et al (2010) Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem 401:318–320
Ferguson RE, Carroll HP, Harris A, Maher ER, Selby PJ, Banks RE (2005) Housekeeping proteins: a preliminary study illustrating some limitations as useful references in protein expression studies. Proteomics 5:566–571
Cardoso TC, Castanheira TLL, Teixeira MCB, Rosa ACG, Hirata KY, Astolphi RD et al (2008) Validation of an immunohistochemistry assay to detect turkey coronavirus: a rapid and simple screening tool for limited resource settings. Poultry Sci 87:1347–1352
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Moss WD (1983) Acid phosphatases. In: Bergmeyer J, Grassi M (eds) Esterases, glycosidases, lyases, ligases, vol 4: methods of enzymatic analysis. Verlag-Chemie, Weinheim, pp 92–106
Mizushima N, Yoshimori T, Ohsumi Y (2010) The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. doi:10.1146/annurev-cellbio-092910-154005
Zhang JY, Pan MH, Sun ZY, Huang SJ, Yu ZS, Liu D et al (2010) The genomic underpinnings of apoptosis in the silkworm, Bombyx mori. BMC Genomics 11:611
Courtiade J, Pauchet Y, Vogel H, Heckel DG (2011) A comprehensive characterization of the caspase gene family in insects from the order Lepidoptera. BMC Genomics 12:357
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−Delta Delta C) method. Methods 25:402–408
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8:741–752
Martinet W, De Meyer GR (2008) Autophagy in atherosclerosis. Curr Atheroscler Rep 10:216–223
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Griswold AJ, Chang KT, Runko AP, Knight MA, Min KT (2008) Sir2 mediates apoptosis through JNK-dependent pathways in Drosophila. Proc Natl Acad Sci USA 105:8673–8678
Goncu E, Parlak O (2011) The influence of juvenile hormone analogue, fenoxycarb on the midgut remodeling in Bombyx mori (L., 1758) (Lepidoptera: Bombycidae) during larval–pupal metamorphosis. Turk J Entomol 35:179–194
Chiarelli R, Agnello M, Roccheri MC (2011) Sea urchin embryos as a model system for studying autophagy induced by cadmium stress. Autophagy 7:1028–1034
Buzgariu W, Chera S, Galliot B (2008) Methods to investigate autophagy during starvation and regeneration in hydra. Methods Enzymol 451:409–437
Shelly S, Lukinova N, Bambina S, Berman A, Cherry S (2009) Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity 30:588–598
Barth JM, Szabad J, Hafen E, Kohler K (2011) Autophagy in Drosophila ovaries is induced by starvation and is required for oogenesis. Cell Death Differ 18(6):915–924
Denton D, Shravage B, Simin R, Baehrecke EH, Kumar S (2010) Larval midgut destruction in Drosophila: not dependent on caspases but suppressed by the loss of autophagy. Autophagy 6:163–165
Dupere-Minier G, Hamelin C, Desharnais P, Bernier J (2004) Apoptotic volume decrease, pH acidification and chloride channel activation during apoptosis requires CD45 expression in HPB-ALL T cells. Apoptosis 9:543–551
Thummel CS (2001) Steroid-triggered death by autophagy. Bioessays 23:677–682
Rybczynski R (2005) Prothoracic hormone. In: Gilbert LI, Iatrou K, Gill SS (eds) Endocrinology, vol 3: comprehensive molecular insect science. Elsevier Pergamon, Oxford, pp 61–123
Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A (2009) Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 16:966–975
Scott RC, Juhasz G, Neufeld TP (2007) Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr Biol 17:1–11
Codogno P, Meijer AJ (2006) Atg5: more than an autophagy factor. Nat Cell Biol 8:1045–1047
Yue ZY, Jin SK, Yang CW, Levine AJ, Heintz N (2003) Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA 100:15077–15082
Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L et al (2006) Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol 8:1124–1132
Neufeld TP, Baehrecke EH (2008) Eating on the fly: function and regulation of autophagy during cell growth, survival and death in Drosophila. Autophagy 4:557–562
Juhasz G, Erdi B, Sass M, Neufeld TP (2007) Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Gene Dev 21:3061–3066
Scott RC, Schuldiner O, Neufeld TP (2004) Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell 7:167–178
Rabinowitz JD, White E (2010) Autophagy and metabolism. Science 330:1344–1348
Parenti P, Giordana B, Sacchi VF, Hanozet GM, Guerritore A (1985) Metabolic activity related to the potassium pump in the midgut of Bombyx mori larvae. J Exp Biol 116:69–78
Jellinger KA, Stadelmann C (2001) Problems of cell death in neurodegeneration and Alzheimer’s disease. J Alzheimers Dis 3:31–40
Guillon-Munos A, van Bemmelen MXP, Clarke PGH (2006) Autophagy can be a killer even in apoptosis-competent cells. Autophagy 2:140–142
Acknowledgments
We thank Professor Congzhao Zhou (University of Science and Technology of China) for providing pET-28b-BmATG8 expression vector. This work was supported by a grant from the Italian Ministry of University and Research (PRIN 2008, protocol 2008SMMCJY) and by FAR 2009–2010 (University of Insubria) to GT, and by grants from the “973” National Basic Research Program of China (No. 2012CB114602), the “863” National High Technology and Research Program of China (No. SQ2010AA1000688007) and Guangdong Province Natural Science Foundation (No. 06105204) to YC and QF.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
E. Franzetti and Z.-J. Huang contributed equally to this work.
An erratum to this article can be found at http://dx.doi.org/10.1007/s10495-011-0693-y.
Rights and permissions
About this article
Cite this article
Franzetti, E., Huang, ZJ., Shi, YX. et al. Autophagy precedes apoptosis during the remodeling of silkworm larval midgut. Apoptosis 17, 305–324 (2012). https://doi.org/10.1007/s10495-011-0675-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-011-0675-0