Abstract
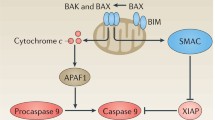
B Cell Lymphoma-2 (Bcl-2) protein suppresses ionizing radiation-induced apoptosis in hemato-lymphoid system. To enhance the survival of irradiated cells, we have compared the effects and mechanism of Bcl-2 and its functional variants, D34A (caspase-3 resistant) and S70E (mimics phosphorylation on S70). Bcl-2 and its mutants were transfected into hematopoietic cell line and assessed for cell survival, clonogenicity and cell cycle perturbations upon exposure to ionizing radiation. The electrostatic potential of BH3 cleft of Bcl-2/mutants and their heterodimerization with Bcl-2 associated X protein (Bax) were computationally evaluated. Correspondingly, these results were verified by co-immunoprecipitation and western blotting. The mutants afford higher radioprotective effect than Bcl-2 in apoptotic and clonogenic assays at D0 (radiation dose at which 37 % cell survival was observed). The computational and functional analysis indicates that mutants have higher propensity to neutralize Bax protein by heterodimerization and have increased caspase-9 suppression capability, which is responsible for enhanced survival. This study implies potential of Bcl-2 mutants or their chemical/peptide mimics to elicit radioprotective effect in cells exposed to radiation.












Similar content being viewed by others
Abbreviations
- A:
-
Alanine
- BH:
-
Bcl-2 Homology domain
- D:
-
Aspartic acid
- E:
-
Glutamic acid
- G:
-
Glycine
- K:
-
Lysine
- L:
-
Leucine
- N:
-
Asparagine
- P:
-
Proline
- Q:
-
Glutamine
- R:
-
Arginine
- S:
-
Serine
- W:
-
Tryptophan
- H:
-
Histidine
- Bcl-2:
-
Wild-type protein
- Variants or Mutants:
-
D34A and S70E
References
Fei P, El-Deiry WS (2003) p53 and radiation responses. Oncogene 22:5774–5783
Verma YK, Gangenahalli GU, Singh VK, Gupta P, Chandra R, Sharma RK, Raj HG (2006) Cell death regulation by B-cell lymphoma protein. Apoptosis 11:459–471
Trisciuoglio D, Gabellini C, Desideri M, Ragazzoni Y, De Luca T, Ziparo E, Del Bufalo D (2011) Involvement of BH4 domain of Bcl-2 in the regulation of HIF-1-mediated VEGF expression in hypoxic tumor cells. Cell Death Differ 18:1024–1035
Raghav PK, Verma YK, Gangenahalli GU (2012) Molecular dynamics simulations of the Bcl-2 protein to predict the structure of its unordered flexible loop domain. J Mol Model 18:1885–1906
Reed JC (1994) Bcl-2 and the regulation of programmed cell death. J Cell Biol 124:1–6
Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM (1999) Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA 96:14943–14948
Meier P, Finch A, Evan G (2000) Apoptosis in development. Nature 407:796–801
Johnson BW, Boise LH (1999) Bcl-2 and caspase inhibitor (zVAD-fmk) cooperate to inhibit tumor necrosis factor-α-induced cell death in a Bcl-2 cleavage independent fashion. J Biol Chem 274:18552–18558
Prasad KN (1995) Handbook of radiobiology. CRC Press, Denver
Maity A, McKenna WG, Muschel RJ (1994) The molecular basis for cell cycle delays following ionizing radiation: a review. Radiother Oncol 31:1–13
Gurudutta GU, Verma YK, Singh VK, Gupta P, Raj HG, Sharma RK, Chandra R (2005) Structural conservation of residues in BH1 and BH2 domains of Bcl-2 family proteins. FEBS Lett 579:3503–3507
Petros AM, Medek A, Nettesheim DG, Kim DH, Yoon HS, Swift K, Matayoshi ED, Oltersdorf T, Fesik SW (2001) Solution structure of the antiapoptotic protein Bcl-2. Proc Natl Acad Sci USA 98:3012–3017
Zoller MJ, Smith M (1987) Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. Methods Enzymol 154:329–350
Gossen M, Bujard H (1995) Efficacy of tetracycline-controlled gene expression is influenced by cell type. Biotechniques 19:213–216
Hillen W, Berens C (1994) Mechanisms underlying expression of tn10 encoded tetracycline resistance. Annu Rev Microbiol 48:345–369
Berninghausen O, Leippe M (1997) Necrosis versus apoptosis as the mechanism of target cell death induced by Entamoeba histolytica. Infect Immun 65:3615–3621
Ohki EC, Tilkins ML, Ciccarone VC, Price PJ (2001) Improving the transfection efficiency of post-mitotic neurons. J Neurosci Methods 112:95–99
Bellosillo B, Villamor N, López-Guillermo A, Marcé S, Bosch F, Campo E, Montserrat E, Colomer D (2002) Spontaneous and drug-induced apoptosis is mediated by conformational changes of Bax and Bak in B-cell chronic lymphocytic leukemia. Blood 100:1810–1816
Arends MJ, Morris RG, Wyllie AH (1990) Apoptosis. The role of the endonuclease. Am J Pathol 136:593–608
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C (2006) Clonogenic assay of cells in vitro. Nat Protoc 1:2315–2319
Sali A, Blundell TL (1993) Comparative protein modeling by satisfaction of spatial restraints. J Mol Biol 234:779–815
Brooks BR, Bruccoleri RE, Swaminathan S, Karplus M (1983) CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J Comput Chem 4:187–217
Rodriguez R, Chinea G, Lopez N, Pons T, Vriend G (1998) Homology modeling, model and software evaluation: three related resources. Bioinformatics 14:523–528
Sharp K, Honig B (1990) Electrostatic interactions in macromolecules: theory and applications. Annu Rev Biophys Chem 19:301–332
Suzuki M, Youle RJ, Tjandra N (2000) Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103:645–654
Ritchie DW (2008) Recent progress and future directions in protein–protein docking. Curr Protein Pept Sci 9:1–15
Ju T, Brewer K, D’Souza A, Cummings RD, Canfield WM (2002) Cloning and expression of human core 1 beta1,3-galactosyltransferase. J Biol Chem 277:178–186
Schandl CA, Li S, Re GG, Fan W, Willingham MC (1999) Mitotic chromosomal Bcl-2: stable expression throughout the cell cycle and association with isolated chromosomes. J Histochem Cytochem 47:139–149
Agarwal N, Tochigi Y, Adhikari AS, Cui S, Cui Y, Iwakuma T (2011) MTBP plays a crucial role in mitotic progression and chromosome segregation. Cell Death Differ 18:1208–1219
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. Cold Spring Harbor Laboratory Press, New York
Prasad S, Yadav R, Kannappan R, Aggarwal BB (2011) Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors: evidence for the role of reactive oxygen species and JNK. J Biol Chem 286:5546–5557
Margolin N, Raybuck SA, Wilson KP (1997) Substrate and inhibitor specificity of interleukin-1β-converting enzyme and related caspases. J Biol Chem 272:7223–7228
Ruvolo PP, Deng X, May W (2001) Phosphorylation of Bcl-2 and regulation of apoptosis. Leukemia 15:515–522
Deng X, Gao F, Flagg T, Anderson J, May WS (2006) Bcl2’s flexible loop domain regulates p53 binding and survival. Mol Cell Biol 26:4421–4434
Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK (2004) Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 116:527–540
Thompson J, Winoto A (2008) Bcl-2 associates with the Nur77 family members and exposes its BH3 pro-apoptotic domain during negative selection. J Exp Med 205:1029–1036
Ohno T, Nakano T, Niibe Y, Tsujii H, Oka K (1998) Bax protein expression correlates with radiation-induced apoptosis in radiation therapy for cervical carcinoma. Cancer 83:103–110
Chen RW, Chuang DM (1999) Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. J Biol Chem 274:6039–6042
Chen CL, Lin CF, Chiang CW, Jan MS, Lin YS (2006) Lithium inhibits ceramide- and etoposide-induced protein phosphatase 2A methylation, Bcl-2 dephosphorylation, caspase-2 activation, and apoptosis. Mol Pharmacol 70:510–517
Dainiak A, Sorba S (1997) Early identification of radiation accident victims for therapy of bone marrow failure. Stem Cells 15:275–285
Yamamoto K, Ichijo H, Korsmeyer SJ (1999) Bcl-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol 19:8469–8478
Dellinger EP, Tellado JM, Soto NE, Ashley SW, Barie PS, Dugernier T, Imrie CW, Johnson CD, Knaebel HP, Laterre PF, Maravi-Poma E, Kissler JJ, Sanchez-Garcia M, Utzolino S (2007) Early antibiotic treatment for severe acute necrotizing pancreatitis: a randomized, double-blind, placebo-controlled study. Ann Surg 245:674–683
Chamond RR, Añón JC, Aguilar CM, Pasadas FG (1999) Apoptosis and disease. Alergol E Inmunol Clin 14:367–374
Acknowledgments
Authors are thankful to Director R. P. Tripathi, INMAS, Delhi, India, for his support. Authors express gratitude to Mr. Rohit Mathur, Mr. J. S. Adhikari, Dr. B. S. Dwarakanath, Ms. Divya Khaitan and Mrs. Namita Kalra for flow cytometric measurements and Mr. Ravi Soni and Dr. Anant Narayan Bhatt for irradiation of cells. Authors are grateful to Prof. Jos Domen, Assistant Research Professor of Medicine, Department of Medicine, Division of Medical Oncology and Transplantation, University of Duke, Durham, North Carolina for gifting Bcl-2 cDNA. In addition, authors acknowledge Dr. Pallavi Gupta for proofreading of the manuscript. Director, Dr. B. R. Ambedkar Centre for Biomedical Research (ACBR), Delhi University, Delhi-110007, India, is appreciated for providing permission to use Accelrys Discovery Studio 2.0 system for 3D-modeling and EP calculations. This work was supported by Defence Research and Development Organisation (DRDO), Government of India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yogesh Kumar Verma and Gurudutta U. Gangenahalli are equally contributed authors.
Rights and permissions
About this article
Cite this article
Verma, Y.K., Raghav, P.K., Raj, H.G. et al. Enhanced heterodimerization of Bax by Bcl-2 mutants improves irradiated cell survival. Apoptosis 18, 212–225 (2013). https://doi.org/10.1007/s10495-012-0780-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-012-0780-8