Abstract
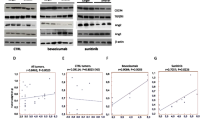
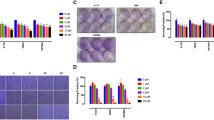
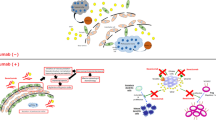
Bevacizumab (BVZ) as an antiangiogenesis therapy leads to a transient therapeutic efficacy in high-grade glioma. However, the proapoptotic potential of BVZ has not been well elucidated, yet. There is also a tumor resistance to BVZ that is linked to post-treatment metalloproteinases and AKT activities. Herein, the association between therapeutic efficacy and putative proapoptotic activity of low-dose BVZ either alone or in combination with a specific inhibitor of AKT called perifosine (PRF), in a glioma model was investigated. BALB/c mice bearing C6 glioma tumor were treated with BVZ and PRF either alone or combined for 13 days (n = 11/group). At the end of treatments, apoptosis, proliferation and vascular density, in the xenografts (3/group) were detected by TUNEL staining, Ki67 and CD31 markers, respectively. Relative levels of cleaved-caspase3, phospho-AKT (Ser473) and matrix metalloproteinase2 (MMP2) were measured using western blotting. PRF and BVZ separately slowed down tumor growth along with the cell apoptosis induction associated with a profound increase in caspase3 activity through an AKT inhibition-related pathway for PRF but not BVZ. Unlike PRF, BVZ significantly increased the intratumor MMP2 and phospho-AKT (Ser473) levels coupled with the slight antiproliferative and significant antivascular effects. Co-administration of PRF and BVZ versus monotherapies potentiated the proapoptotic effects and reversed the BVZ-induced upregulation of phospho-AKT (Ser473) and MMP2 levels in C6 xenografts, leading to the optimal antiproliferative activity and tumor growth regression and longer survival. In conclusion, BVZ plus PRF renders a paramount proapoptotic effect, leading to a major therapeutic efficacy and might be a new substitute for GBM therapy in the clinic.




Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol (Berl) 114(2):97–109
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN (2006) Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 66(16):7843–7848
Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, Hoffman RM, Kerbel RS (2009) Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res 69(18):7243–7251
Cohen MH, Shen YL, Keegan P, Pazdur R (2009) FDA drug approval summary: bevacizumab (Avastin®) as treatment of recurrent glioblastoma multiforme. Oncologist 14(11):1131–1138
Ramezani S, Vousooghi N, Kapourchali FR, Hadjighasem M, Hayat P, Amini N, Joghataei MT (2017) Rolipram potentiates bevacizumab-induced cell death in human glioblastoma stem-like cells. Life Sci 173:11–19
Wang L-L, Hu R-C, Dai A-G, Tan S-X (2015) Bevacizumab induces A549 cell apoptosis through the mechanism of endoplasmic reticulum stress in vitro. Int J Clin Exp Pathol 8(5):5291
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307(5706):58–62
de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, Conrad CA (2010) Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro-oncology 12(3):233–242
Iwamoto F, Abrey L, Beal K, Gutin P, Rosenblum M, Reuter V, DeAngelis L, Lassman A (2009) Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology 73(15):1200–1206
Takano S, Mashiko R, Osuka S, Ishikawa E, Ohneda O, Matsumura A (2010) Detection of failure of bevacizumab treatment for malignant glioma based on urinary matrix metalloproteinase activity. Brain Tumor Pathol 27(2):89–94
Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD, Phillips JJ, Cheresh DA (2012) VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer cell 22(1):21–35
Simon T, Coquerel B, Petit A, Kassim Y, Demange E, Le Cerf D, Perrot V, Vannier J-P (2014) Direct effect of bevacizumab on glioblastoma cell lines in vitro. Neuromolecular Med 16(4):752–771
von Baumgarten L, Brucker D, Tirniceru A, Kienast Y, Grau S, Burgold S, Herms J, Winkler F (2011) Bevacizumab has differential and dose-dependent effects on glioma blood vessels and tumor cells. Clin Cancer Res 17(19):6192–6205
Nitulescu GM, Margina D, Juzenas P, Peng Q, Olaru OT, Saloustros E, Fenga C, Spandidos DΑ, Libra M, Tsatsakis AM (2016) Akt inhibitors in cancer treatment: the long journey from drug discovery to clinical use (review). Int J Oncol 48(3):869–885
Sudaka A, Susini A, Nigro CL, Fischel J-L, Toussan N, Formento P, Tonissi F, Lattanzio L, Russi E, Etienne-Grimaldi M-C (2013) Combination of bevacizumab and irradiation on uveal melanoma: an in vitro and in vivo preclinical study. Invest New Drugs 31(1):59–65
Rapisarda A, Hollingshead M, Uranchimeg B, Bonomi CA, Borgel SD, Carter JP, Gehrs B, Raffeld M, Kinders RJ, Parchment R (2009) Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol Cancer Ther 8(7):1867–1877
Tsou H-K, Chen H-T, Hung Y-H, Chang C-H, Li T-M, Fong Y-C, Tang C-H (2013) HGF and c-Met interaction promotes migration in human chondrosarcoma cells. PLoS ONE 8(1):e53974
Fayard E, Tintignac LA, Baudry A, Hemmings BA (2005) Protein kinase B/Akt at a glance. J Cell Sci 118(24):5675–5678
Grzmil M, Hemmings BA (2010) Deregulated signalling networks in human brain tumours. Biochimica et Biophysica Acta (BBA) 1804(3):476–483
Bisgin A, Kargi A, Yalcin AD, Aydin C, Ekinci D, Savas B, Sanlioglu S (2012) Increased serum sTRAIL levels were correlated with survival in bevacizumab-treated metastatic colon cancer. BMC Cancer 12(1):58
Fulda S, Debatin K (2006) Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25(34):4798–4811
Haupt S, Berger M, Goldberg Z, Haupt Y (2003) Apoptosis-the p53 network. J Cell Sci 116(20):4077–4085
Ferrara N (2005) VEGF as a therapeutic target in cancer. the International Society for Cellular 69(Suppl. 3):11–16
Ferrara N, Hillan KJ, Novotny W (2005) Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun 333(2):328–335
Claesson-Welsh L, Welsh M (2013) VEGFA and tumour angiogenesis. J Intern Med 273(2):114–127
Karar J, Maity A (2011) PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci 4:51
Soler A, Angulo-Urarte A, Graupera M (2015) PI3K at the crossroads of tumor angiogenesis signaling pathways. Mol Cell Oncol 2(2):e975624
Fukumura D, Jain RK (2007) Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res 74(2):72–84
Figg WD, Monga M, Headlee D, Shah A, Chau CH, Peer C, Messman R, Elsayed YA, Murgo AJ, Melillo G (2014) A phase I and pharmacokinetic study of oral perifosine with different loading schedules in patients with refractory neoplasms. Cancer Chemother Pharmacol 74(5):955–967
Gojo I, Perl A, Luger S, Baer MR, Norsworthy KJ, Bauer KS, Tidwell M, Fleckinger S, Carroll M, Sausville EA (2013) Phase I study of UCN-01 and perifosine in patients with relapsed and refractory acute leukemias and high-risk myelodysplastic syndrome. Invest New Drugs 31(5):1217–1227
Richardson PG, Wolf J, Jakubowiak A, Zonder J, Lonial S, Irwin D, Densmore J, Krishnan A, Raje N, Bar M (2011) Perifosine plus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma previously treated with bortezomib: results of a multicenter phase I/II trial. J Clin Oncol 29(32):4243–4249
Acknowledgements
The authors would like to thank the all staffs of Cellular and Molecular Research Center in Iran University of Medical Sciences, who helped us in performing the animal care and treatment processes.
Funding
This project was funded by Iran University of Medical Sciences (Grant No. 24164) and Iran National Science Foundation (Grant No. 92035151).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Ramezani, S., Vousooghi, N., Ramezani Kapourchali, F. et al. Perifosine enhances bevacizumab-induced apoptosis and therapeutic efficacy by targeting PI3K/AKT pathway in a glioblastoma heterotopic model. Apoptosis 22, 1025–1034 (2017). https://doi.org/10.1007/s10495-017-1382-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1382-2