Abstract
The heritability of sensation seeking is investigated in an extended twin design, including mono- and dizygotic twins and their siblings. Besides a comparison of the phenotypic resemblance between monozygotic twins and dizygotic twins, the design allows for an explicit test of the assumption that results from twins may be generalized to the singleton population. Secondly, the design offers the opportunity to investigate to what extent the influence of common environment is the same for males and females and for twins and siblings, i.e. allowing for explicit tests of a special twin environment and of a sex-specific common environment. The results indicate that individual variation in sensation seeking is heritable, with few differences between males and females in heritability estimates for the sensation seeking dimensions. In contrast to prior studies, evidence is found for common environmental influences for thrill and adventure seeking in males, and experience seeking and boredom susceptibility in females. Evidence for a special twin environment was limited to boredom susceptibility in females.
Similar content being viewed by others
Introduction
Sensation seeking can be defined as an individuals’ need for varied, novel, and complex sensations and experiences (Zuckerman, 1979). It manifests itself in a non-conforming lifestyle, in the search for new experiences (Aron and Aron, 1997), the capacity to inhibit behavior in service of social adaption (Zuckerman, 1993) and engagement in risk full activities. The first investigations of sensation seeking focused on its relation to the concept of an optimal level of stimulation and arousal (Berlyne and Madsen, 1973; Zuckerman, 1969). People differ in their optimal level of stimulation, and sensation seeking behavior can be used to increase this level. The idea of an optimal level of stimulation and arousal was incorporated in a theory that attempted to explain individual differences in response to sensory deprivation (Zuckerman, 1969). Since then, research on sensation seeking found that the personality trait is associated with lifestyle factors such as smoking, alcohol consumption, using other drugs (Zuckerman, 1994), and sports participation (Franken et al., 1994; Jack and Ronan, 1998). Potgieter and Bisschoff (1990), for instance, concluded that sensation seeking may serve as a possible underlying explanation for the motivation of individuals to participate in high-risk versus low-risk sports. More specifically, it has been found that sensation seeking is positively related to participation in high- and medium-risk sports, and negatively with low-risk sports. Sensation seeking is also associated with preferences for unfamiliar foods (Pliner and Melo, 1997), drunk driving (Arnett, 1990), hazardous sex practices (Kalichman and Rompa, 1995), perceived funniness (Lourey and McLachlan, 2003), curiosity (Gottfried et al., 1994) and intelligence (Raine et al., 2002). Recently it was found by Joireman et al. (2003) that sensation seeking may predict the desire to engage in physical and verbal aggression.
In addition to the research on how individual differences in sensation seeking, and its components, are correlated with individual differences in other traits there has been a focus on the determinants of the interindividual differences in terms of genetic and biological influences. A large body of research demonstrates that interindividual differences in sensation seeking have a biological basis (Zuckerman, 2002). More specifically, genetic analyses (Eysenck, 1983; Fulker et al., 1980; Hur and Bouchard, 1997; Koopmans et al., 1995) showed that the genetic effects on sensation seeking are accounting for a large part of the variance (ranging from 34% to 69%). High heritability of a personality trait does, however, not exclude the influence of common environmental factors. Indeed, biographical factors, religion, parental stimulation, and other (parental) family characteristics like socio-economic status (SES) have also been shown to have an effect on sensation seeking (Feij and Taris, 2005; Zuckerman, 1994).
In genetically informative designs, environmental factors are usually separated into common environmental factors that are shared by all members of a family (or pedigree) and unique environmental factors that apply only to a single family member. Religion and parental SES and rearing style are usually considered part of the common environment. Results like those of Zuckerman (1994) and Feij and Taris (2005), therefore, seem to suggest that common environmental factors should contribute to interindividual variance in sensation seeking. This is, however, not supported by the genetic analyses of Eysenck (1983), Koopmans et al. (1995), and Hur and Bouchard (1997). These studies found effects of environmental variables on sensation seeking, but these were unique to family members, and thus not due to the common environment. However, even the sample size of the largest study (Koopmans et al., 1995) which included 1591 families, is not enough to detect a small amount (10–20%) of common environmental variance with a power of 0.80 under an MZ/DZ ratio of 1/1 (Posthuma and Boomsma, 2000, p.157).
In the present study we aim to maximize the statistical power to detect the influence of common environment by (1) using a very large sample, and (2) including non-twin siblings in the design (See Figure 1). Such an extension of the standard twin design is not only optimal for future gene detection (Dolan et al., 1999), but also for the estimation of the genetic and environmental influences. More explicitly, Posthuma and Boomsma (2000) showed that, for finding a specific amount of common environmental variance under an MZ/DZ ratio of 1/1 with a statistical power of 0.80, extending the twin design with one sibling would require just half the number of complete families.
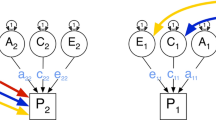
Extended twin design. Graphical representation of the variance decomposition of the covariance matrix into A, C and E components. Correlations of A components are constrained to the value of 1 for MZ, and to 0.5 for DZ, and twin-sib pairs. The interindividual correlations of the C components (R c(t♂,t♀), R c(t♂,s♂), R c(t♀,s♀), R c(t♂,s♀) and R c(s♂,t♀)) may be estimated or constrained to 1 for MZ, DZ, and twin-sib pairs, and the E components are left uncorrelated across family members. The variances of all latent variables are constrained to 1, and the factor loadings (a m, c m, e m, a f, c f and e f) are estimated, and constrained to be equal within males and females. Although means are estimated they are not included in the figure. Age was included in the model, but is not drawn explicitly, in order to keep the figure orderly.
In addition to an increase in statistical power, two important aspects of the extension of the twin design are that it allows for an explicit test of (1) whether the twins and singletons come from the same population, by comparing means and variances of twins and singletons, and (2) whether there is a special twin environment, by comparing the twin–twin covariance to twin–singleton covariance.
As a main innovation over previous applications of the extended twin design (Posthuma and Boomsma, 1999, 2000; Posthuma et al., 2000), this study estimates the sex-specific and twin-sib specific common environmental correlations. The test of sex-specific common environmental factors is realized by comparing common environmental correlation (R c(t♂, t♀)) in the opposite-sex twins to one. Likewise, if the twin-sib specific common environmental correlations, i.e. R c(t♂, s♂) and R c(t♀, s♀), differ from one, this indicates that twins share more of their environment with each other, than they do with an additional sib.
A potential problem implied by the inclusion of non-twin siblings is that these additional family members differ in age from their twin brother(s) or sister(s). These within family age differences may consequently confound the tests of the common environmental correlations. In our sample, most twins and their siblings have been measured more than once over time, providing the opportunity to select a subset from the whole data set in which age differences between twins and their sibs within families are minimized as part of the design.
The main aims of this paper are to first test for the existence of common environmental influences in a design with increased statistical power to detect such influences, and secondly, given common environmental influences, to test for the existence of a (sex-specific) special twin environment.
Method
Subjects
The data for this study were taken from the Netherlands Twin Register (NTR, see Boomsma et al., 2002, for a detailed description). Participants were adolescent and adult twins and their siblings. We selected twin pairs and, if possible one additional sibling who participated in at least one of the 5 biannual surveys between the years 1991 and 2000. For the first waves of the NTR data collection twins and their parents were invited to participate. Starting at wave 3 and onwards, additional siblings were approached as well. On each wave the subjects filled out various items on zygosity, health, alcohol and tobacco use, sports participation, and personality. No items on sensation seeking were administered in the third wave, so data from wave 1, 2, 4 and 5 were used in this study.
The criterion for selection of the measurement occasion that was used for these analyses was to minimize the age differences within families of the twins and their sibling. For example, if the age of the twins and their sibling was for a specific family, respectively, 24 and 16 years at the first occasion, the selection resulted in data from the first occasion for the twins, and data from the fifth occasion for their sibling. If there were missing data or if the age difference between twins and their sibling was too large, so that no ideal selection of the available measurement occasions could be made, measurement occasions were selected that minimized the age differences. Wherever it was possible, twin data from the same occasion were selected. Furthermore, if more than two ‘ideal’ combinations were found, that combination was chosen for which the age of the twins was the highest. For example, if the age of the twins and their sibling was, respectively, 24 years and 18 years at the first occasion, there are two ideal combinations (twins at the first and sibling at the fourth occasion versus twins at the second and sibling at the fifth occasion). Both combinations give a zero age difference between twins and their sibling. Given the criterion described before, the last combination was chosen in these cases. Footnote 1
Zygosity of the twins was determined using items about physical similarity and the frequency of confusion of the twins by family and strangers (Goldsmith et al., 1991; Magnus et al., 1983). For 695 same-sex twin pairs, zygosity was assessed by typing blood group and/or DNA polymorphisms. It was found that classification based on these questionnaire data compared with DNA fingerprinting was correct for more than 96% of the cases.
The resulting sample consists of a total of 9220 individuals: 5391 females and 3829 males from 4281 families. We were able to include one additional non-twin sibling for 1343 families. There were 726 sisters, and 617 brothers; half-siblings were not included. These non-twin siblings increased the number of sib pairs with 2561 (more than 50%). As a consequence of the inclusion of additional siblings the monozygotic (MZ)-pair to dizygotic (DZ)-pair ratio decreases from 0.80 (1608/2000) to 0.35 (1608/4561). Table I provides information on the number of different pairs and triplets that could be formed for each zygosity group. Of the 1343 non-twin siblings, 23% were younger than the twins, and 77% were older. Average age of twins was 25.3 years and of their sibling 29.0 years with standard deviation of respectively, 10.9 and 10.4.
Measures
Sensation seeking is thought to consist of four separate components each encompassing different ways of sensation seeking and arousal (Zuckerman, 1979, 1994). The first of the four components, ‘Thrill and adventure seeking’ (TAS), is defined as the desire to engage in various somewhat risky sports or activities involving speed, adventure, defiance of gravity, or unusual sensations. The second component, ‘Experience seeking’ (ES), is defined as the (desired) experience through the mind and the senses, through art, travel, food, dress, and through non-conformist friends and groups. ‘Disinhibition’ (DIS) is defined as the desire to find release through other exciting people, disinhibited parties, and sexual variety; and finally, ‘Boredom susceptibility’ (BS) is the experienced boredom and an intolerance for repetition of any kind, monotonous conditions and boring people, and restlessness when alone in familiar surroundings. Sensation seeking was measured with the Dutch translation of Zuckerman’s Sensation Seeking Scale (SSS), form IV (Feij and Zuilen, 1984; Zuckerman, 1971). The Dutch questionnaire consists of 51 forced choice items measuring TAS, ES, DIS and BS. Each item is measured on a 5-point Likert scale (ranging from completely agree to completely disagree), and loads uniquely on one of the four factors. The four scales of the SSS have a good factor structure, reasonably high internal consistencies ranging from 0.72 to 0.81, and good construct validity (Feij et al., 1997, 1982). The internal consistencies of sensation seeking in the present data set range from 0.59 for ES to 0.83 for TAS.
Missing Data and Description of Analyses
An essential characteristic of the extended twin-design is the presence of structural missing data. Some families consist of twins and an additional younger or older sibling, while other families consist of one or two twins. A second source of structural missing data comes from the use of sex specific models. Differentiation needs to be made between male and female non-twin siblings, and given the design with one non-twin sibling, families can include only a male or a female non-twin sibling. We used full-information maximum likelihood estimation for raw data, as implemented in the software package Mx (Neale et al., 1999), for parameter estimation which implies the assumption that the underlying missing data mechanism is Missing At Random (Little and Rubin, 1987). It is assumed that all families include four persons, two twins, and a male and female sibling. For families that do not include non-twin siblings the corresponding data points are made missing; for families that include a non-twin sibling, the male or female sibling data points are made missing. Since families may include only one additional sibling (male or female), the covariance between these entities is non-existent (i.e. there are no non-twin male and non-twin female pairs). This has been accounted for in the present analyses by constraining the corresponding elements in the covariance matrix at zero. All MX-scripts can be downloaded through the internet (see Footnote 1).
The first analysis involved fitting a saturated model to the raw data in a 6-group model (MZM, DZM, MZF, DZF, DOSMF, DOSFM, and their sibs). This saturated model is used to test the standard assumptions of the twin design. These assumptions include equality of means and variances for males and females, for first and second born twins and sibs, and for mono- and dizygotic twins (See Appendix 1). The second step consists of the analyses of the genetic models. A full decomposition in additive genetic (A), common environmental (C), and unique factors (E), as presented in Figure 1 is carried out.
Including opposite sex pairs in the analysis allows for explicit tests of the hypotheses of whether the same environmental factors operate in males and females, and in twins and siblings. In the present analyses the correlations that are used to test these hypotheses, R c(t♂,t♀), R c(t♂,s♀), R c(t♀,s♂), R c(t♂,s♂), R c(t♀,s♀), are estimated. The environmental correlations between same sex twins (R c(t♂,t♂), R c(t♀,t♀)) are constrained to the value of 1 to identify the model.
Although within family differences in age are minimized by the design, between family differences in age are still present. Because age was expected to impact on all four variables, these differences in age may act to increase the estimate of the common environment. Therefore, between family difference in age on the mean family score will be accounted for by including age as a covariate.
Goodness of fit testing was based on the likelihood ratio test. Given the number of nested models that were tested against each other, and the large N, we tested each model in the sequence at a significance level of α=0.01.
Results
Descriptive Statistics and Test of Assumptions
Table II presents descriptive statistics of the four subscales separately for males and females. Males score higher than females on all four subscales. On average, the mean of males was half a standard deviation higher than the mean of females, with larger differences for TAS and DIS than for BS and ES. The last column of Table II shows the phenotypic correlations with age. As expected age has a negative correlation with all four subscales.
The first step in the analysis was to test the assumptions of the extended twin design. The results imply that for none of the four scales the assumptions of the extended twin design have been violated (See Table III). That is, none of the restrictions implied by these assumptions led to a significant decrease in model fit at an α-level of 0.01. So it may be concluded that the means and variances within each sex are equal for the different zygosity groups, for first and second-born twins and that no significant differences exist between the means and variances of twins and singletons. Table IV provides the twin–twin and twin-sibling correlations based on the saturated model corrected for age for each scale.
Heritability of Sensation Seeking
The pattern of twin and sibling correlations presented in Table IV, with the MZ correlations being approximately twice the DZ, DOS, and TS correlations, suggest that a reasonably large amount of the individual differences in sensation seeking may be attributed to additive genetic factors.
The results of the genetic analyses of the four scales are presented in Table V. The likelihood ratio test (i.e. Δχ2) was used for testing of the genetic models against the restricted saturated model. In the best fitting model for TAS the estimate of the common environment for females is not significantly different from zero, and as a consequence common environmental correlations containing a female are non-existent. Broad sense heritability of TAS is equal to 34% for males and to 62% for females. Common environmental influences account for 21% of the male variance. Based on the perfect correlation between the environments male twins and their brothers, it can be concluded that there is no special twin environment for males.
The best fitting genetic model for DIS essentially has all common environmental components constrained to zero. Based on this model, we infer the heritability for DIS to be 59% for males, and 52% for females, and no common environmental effects for either sex.
The results for BS need a little more explanation. While three of the four common environmental correlations were not significantly different from 1, the female twin sibling common environmental correlation was estimated as −0.46. This would indicate the rather strange result that those influences that effect female twins have an opposite effect on their non-twin sister. Although this might indeed be the case, another more plausible explanation for the negative correlation might be that the model estimates are overfitting the data (cf. Prescott et al., 2004) because of the low phenotypic correlation between female twins and their sister (R c(t♀,s♀)=0.08, see Table IV). In order to account for this, R c(t♀,s♀) is bounded to be positive in the final model for BS, and is subsequently estimated, at its lower bound of zero. In the final model for BS, the heritability of BS was estimated at 48% for males and 29% for females. There was no influence of common environment in males, whereas common environmental effects account for 18% of the total variance in females. The environmental effects in female twins and female singletons were found to be uncorrelated. More explicitly, given this model, the common environmental effects were different for female twins and their non-twin sisters.
The best fitting model for ES is similar to that of BS, with male C not being significantly different from zero. However, the female twin sibling environmental correlation could be constrained to the value of 1. Based on this final model we report a heritability of 60% for males and 42% for females, with common environmental effects accounting for 13% of the female phenotypic variance. Based on the perfect correlation between the environments female twins and their sister, it can be concluded that no special twin environment exists for females regarding ES.
Discussion
In this article the heritability of sensation seeking, as measured by the Dutch translation of Zuckerman’s Sensation Seeking Scale, is investigated in an extended twin design. Within both sexes individual variation in sensation seeking is shown to be heritable and environmental influences are found to be mainly individual specific. The highest heritability is found for male experience seeking and disinhibition (respectively, 60% and 59%), and the lowest for female boredom susceptibility (29%) and male thrill and adventure seeking (34%). In contrast to prior studies, with much lower statistical power, we find evidence for common environmental influences for male thrill and adventure seeking (21% of phenotypic variance) and female experience seeking and boredom susceptibility (respectively, 13% and 18%). There is evidence for a special twin environment for females regarding boredom susceptibility.
The results of our study may have important implications for conclusions regarding the role of the shared environment as reported by others (e.g. Feij and Taris, 2005; Zuckerman, 1994). The recent study by Feij and Taris (2005), for instance, reported significant correlations between disinhibition and factors that are usually regarded to be part of the common environment, like the parental family environment and parenting behaviors. Even with its large statistical power, the current study rejected the hypothesis of common environmental effects on disinhibition. The correlations reported by Feij and Taris thus must reflect unique environmental effects. It has indeed been shown by these authors that parenting styles, for example, are differently perceived by different children of the same family. The combination of our results with those of Feij and Taris are in correspondence with the notion of Zuckerman (1994) that, the lack of influence of a shared environment does not mean that the parental family environment cannot have specific effects on siblings in the same family.
In summary, the results of this study once again show that sensation seeking belongs to the category of highly heritable personality characteristics. However, though small in comparison to genetic influences, common environment influences also impact on this trait. Using an extended twin design it has been found that the common environment contributes to individual differences between males in thrill and adventure seeking, and between females in both boredom susceptibility and experience seeking. This paper additionally serves as a further illustration of the benefits of extending the standard twin design with just one sibling, and it shows how the common environmental correlations can be used to test the existence of a sex-specific special twin environment.
Notes
SPSS-script containing the full selection procedure for our specific data set and Mx scripts for the models described in this paper are available at the Mx Scripts Library at: http://www.psy.vu.nl/mxbib
References
Arnett J. (1990). Drunk driving, sensation seeking, and egocentrism among adolescents. Pers. Individ. Dif. 11: 541–546
Aron E. N., Aron A. (1997). Sensory-processing sensitivity and its relation to introversion and emotionality. J. Pers. Soc. Psychol. 73: 345–368
Berlyne D. E., Madsen K. B. (1973). Pleasure, and reward preference: Their nature, determinants, and role in behavior. New York, Academic Press
Boomsma D. I., Vink J. M., van Beijsterveldt T. C. E. M., de Geus E. J. C., Beem A. L., Mulder E. J. C. M. et al. (2002). Netherlands twin register: A focus on longitudinal research. Twin Res. 5: 401–406
Dolan C. V., Boomsma D. I., Neale M. C. (1999). A note on the power provided by sibships of size 3 and 4 in genetic covariance modeling of a codominant QTL. Behav. Genet. 29: 163–170
Eysenck H. J. (1983). A biometrical-genetical analysis of impulsive and sensation seeking behavior. In: Zuckerman M. (eds) Biological bases of sensation seeking, impulsivity, and anxiety. Hillside, NJ, Erlbaum, pp. 1–27
Feij J. A., Dekker P. H., Koopmans J. R., Boomsma D. I. (1997). Nieuwe normen en stabiliteits gegevens voor de Spanningsbehoeftelijst (SBL). Nederlands tijdschrift voor de psychologie 52: 131–134
Feij, J. A. and Taris, T. W. (2005). Beyond the genetic basis of sensation seeking: The influences of birth order, family size and parenting styles. Submitted for publication
Feij J. A., van Zuilen R. W. (1984). Handleiding bij de spanningsbehoeftelijst (SBL). Lisse, Swets & Zeitlinger
Feij J. A., van Zuilen R. W., Gazendam A. (1982). De ontwikkeling van een Nederlandse vragenlijst voor sensation seeking: de Spanningsbehoeftelijst (SBL). Gedrag 10: 364–383
Franken R. E., Hill R., Kierstead J. (1994). Sport Interest As Predicted by the Personality Measures of Competitiveness, Mastery, Instrumentality, Expressivity, and Sensation Seeking. Pers. Individ. Dif. 17: 467–476
Fulker D. W., Eysenck S. B. G., Zuckerman M. (1980). A genetic and environmental-analysis of sensation seeking. J. Res. Pers. 14: 261–281
Goldsmith H. H., Rieserdanner L. A., Briggs S. (1991). Evaluating convergent and discriminant validity of temperament questionnaires for preschoolers, toddlers, and infants. Dev. Psychol. 27: 566–579
Gottfried A. W., Gottfried A. E., Bathurst K., Guerin D. (1994). Gifted IQ: Early developmental aspects. Plenum Publishing
Hur Y. M., Bouchard T. J. (1997). The genetic correlation between impulsivity and sensation seeking traits. Behav. Genet. 27: 455–463
Jack S. J., Ronan K. R. (1998). Sensation seeking among high- and low-risk sports participants. Pers. Individ. Dif. 25: 1063–1083
Joireman J., Anderson J., Strathman A. (2003). The aggression paradox: Understanding links among aggression, sensation seeking, and the consideration of future consequences. J. Pers. Soc. Psychol. 84: 1287–1302
Kalichman S. C., Rompa D. (1995). Sensation seeking and sexual compulsivity scales – reliability, validity, and predicting HIV risk behavior. J. Pers. Assess. 65: 586–601
Koopmans J. R., Boomsma D. I., Heath A. C., van Doornen L. J. P. (1995). A multivariate genetic-analysis of sensation seeking. Behav. Genet. 25: 349–356
Little R. J. A., Rubin D. B. (1987). Statistical analysis with missing data. New York, Wiley
Lourey E., McLachlan A. (2003). Elements of sensation seeking and their relationship with two aspects of humor appreciation – perceived funniness and overt expression. Pers. Individ. Dif. 35: 277–287
Magnus P., Berg K., Nance W. E. (1983). Predicting zygosity in norwegian twin pairs born 1915–1960. Clin. Genet. 24: 103–112
Neale, M. C., Boker, S. M., Xie, G. and Maes, H. H. (1999). Mx: Statistical modeling (5th ed.) [Computer software]. VCU Box 900126, Richmond, VA 23298: Department of Psychiatry
Pliner P., Melo N. (1997). Food neophobia in humans: Effects of manipulated arousal and individual differences in sensation seeking. Physiol. Behav. 61: 331–335
Posthuma D., Boomsma D. I. (1999). Adding non-twin siblings to increase power. Behav. Genet. 29: 366
Posthuma D., Boomsma D. I. (2000). A note on the statistical power in extended twin designs. Behav. Genet. 30: 147–158
Posthuma D., de Geus E. J. C., Neale M. C., Pol H. E. H., Baare W. E. C., Kahn R. S. et al. (2000). Multivariate genetic analysis of brain structure in an extended twin design. Behav. Genet. 30: 311–319
Potgieter J., Bisschoff F. (1990). Sensation seeking among medium-risk and low-risk sports participants. Percept. Mot. Skills 71: 1203–1206
Prescott C. A., Cross R. J., Kuhn J. W., Horn J. L., Kendler K. S. (2004). Is risk for alcoholism mediated by individual differences in drinking motivations?. Alcohol. Clin. Exp. Res. 28: 29–39
Raine A., Reynolds C., Venables P. H., Mednick S. A. (2002). Stimulation seeking and intelligence: A prospective longitudinal study. J. Pers. Soc. Psychol. 82: 663–674
Zuckerman M. (1969). Theoretical formulations. In: Zubeck J. P. (eds) Sensory deprivation: Fifteen years of research. New York, Appleton-Century, pp. 407–432
Zuckerman M. (1971). Dimensions of sensation seeking. J. Consult. Clin. Psychol. 36: 45–52
Zuckerman M. (1979). Sensation seeking: Beyond the optimal level of arousal. Hillsdal, NJ, Erlbaum
Zuckerman M. (1993). P-impulsive sensation seeking and its behavioral, psychophysiological and biochemical correlates. Neuropsychobiology 28: 30–36
Zuckerman M. (1994). Behavioral expressions and biosocial bases of sensation seeking. New York, Cambridge University Press
Zuckerman M. (2002). Genetics of sensation seeking. In: Benjamin J., Ebstein R. P., Belmake R. H. (eds) Molecular genetics and the human personality. Washington, DC, American Psychiatric Association, pp. 193–210
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by grants from the Netherlands Organization for Scientific research. [NWO 904-61.193 ‘Resolving cause and effect in the association between regular exercise and psychological well-being’ and NWO 575-25-006 ‘Database Twin register’].
Appendix 1
Appendix 1
The test of the assumptions of the twin design has 89 degrees freedom divided into the following nested sequence of models: First it was tested whether the age effects could be constrained to be equal across all groups and subject (23 df). Second, the means and covariances of the two DOS groups were constrained to be equal (11 df). Third, it was tested whether the means of male twins and siblings could be constrained to be equal within each sex (10 df), and whether the means of female twins and siblings could be constrained to be equal within each sex (10 df). Fourth, it was tested whether the variances of male and female twins and siblings could be constrained to be equal within each sex (2 times 9 df). This model thus estimates, for each scale, just one mean and one variance for all males, and one mean and variance for all females. Finally, the covariances of corresponding pairs were constrained to be equal (17 df). That is, this model constrains the same sex male twin-sibling covariances to be equal, as well as the same sex female twin-sibling covariances, and the opposite sex twin-sibling covariances.
Rights and permissions
About this article
Cite this article
Stoel, R., De Geus, E. & Boomsma, D. Genetic Analysis of Sensation Seeking with an Extended Twin Design. Behav Genet 36, 229–237 (2006). https://doi.org/10.1007/s10519-005-9028-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-005-9028-5