Abstract
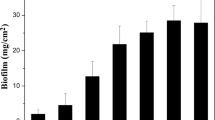
Although biofilms produced by various Leuconostoc sp. are economically important as contaminants of sugar processing plants, very few studies are available on these systems. Twelve strains of Leuconostoc citreum and L. mesenteroides that produce a variety of extracellular glucans were compared for their capacity to produce biofilms. 16s rRNA sequence analysis was used to confirm the species identity of these strains, which included four isolates of L. mesenteroides, five isolates of L. citreum, and three glucansucrase mutants of L. citreum strain NRRL B-1355. Strains identified as L. mesenteroides produce glucans that are generally similar to commercial dextran. Nevertheless, these strains differed widely in their capacity to form biofilms, with densities ranging from 2.7 to 6.1 log cfu/cm2. L. citreum strains and their derivatives produce a variety of glucans. These strains exhibited biofilm densities ranging from 2.5 to 5.9 log cfu/cm2. Thus, biofilm-forming capacity varied widely on a strain-specific basis in both species. The types of polysaccharides produced did not appear to affect the ability to form biofilms.


Similar content being viewed by others
References
Altschul SF, Madden T, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Badel S, Laroche C, Gardarin C, Bernardi T, Michaud P (2008) New method showing the influence of matrix components in Leuconostoc mesenteroides biofilm formation. Appl Biochem Biotechnol 151:364–370
Bischoff KM, Skinner-Nemec KA, Leathers TD (2007) Antimicrobial susceptibility of Lactobacillus species isolated from commercial ethanol plants. J Ind Microbiol Biotechnol 34:739–744
Christensen BE (1989) The role of extracellular polysaccharides in biofilms. J Bacteriol 10:181–202
Cote GL (2002) Alternan. Biopolymers, chap 12. In: Vandamme EJ, DeBaets S, Steinbuchel A (eds) Polysaccharides I: polysaccharides from prokaryotes, vol 5. Wiley-VCH, Weinheim, pp 323–350
Cote GL, Leathers TD (2005) A method for surveying and classifying Leuconostoc spp. glucansucrases according to strain-dependent acceptor product patterns. J Indus Microbiol Biotechnol 32:53–60
Cote GL, Leathers TD (2009) Insoluble glucans from planktonic and biofilm cultures of mutants of Leuconostoc mesenteroides NRRL B-1355. Appl Microbiol Biotechnol 82:149–154
Cote GL, Robyt JF (1982) Isolation and partial characterization of an extracellular glucansucrase from L. mesenteroides NRRL B-1355 that synthesizes an alternating (1 → 6), (1 → 3)-d-glucan. Carbohyd Res 101:57–74
Heersink J (2003) Basic biofilm analytical methods. In: Hamilton M, Heersink J, Buckingham-Meyer K, Goeres D (eds) The biofilm laboratory, step-by-step protocols for experimental design, analysis, and data interpretation. Cytergy Publishing, Bozeman, MT, pp 16–23
Jahn A, Nielsen PH (1998) Cell biomass and exopolymer composition in sewer biofilms. Water Sci Technol 37:17–24
Jeanes A (1977) Dextrans and pullulans: industrially significant α-d-glucans. In: Sandford PA, Laskin A (eds) ACS Symposium series no. 45, Extracellular microbial polysaccharides. American Chemical Society, Washington, DC, pp 284–298
Jeanes A, Haynes WC, Wilham CA, Rankin JC, Melvin EH, Austin MJ, Cluskey JE, Fisher BE, Tsuchiya JH, Rist CE (1954) Characterization and classification of dextrans from ninety-six strains of bacteria. J Am Chem Soc 76:5041–5052
Kim D-S, Fogler HS (1999) The effects of exopolymers on cell morphology and culturability of Leuconostoc mesenteroides during starvation. Appl Microbiol Biotechnol 52:839–844
Kim D-S, Fogler HS (2000) Biomass evolution in porous media and its effects on permeability under starvation conditions. Biotechnol Bioengin 69:47–56
Leathers TD (2002) Dextran. Biopolymers, chap 12. In: Vandamme EJ, De Baets S, Steinbuchel A (eds) Polysaccharides I: polysaccharides from prokaryotes, vol 5. Wiley-VCH, Weinhein, pp 299–321
Leathers TD, Cote GL (2008) Biofilm formation by exopolysaccharide mutants of Leuconostoc mesenteroides strain NRRL B-1355. Appl Microbiol Biotechnol 78:1025–1031
Leathers TD, Hayman GT, Cote GL (1995) Rapid screening of Leuconostoc mesenteroides mutants for elevated proportions of alternan to dextran. Curr Microbiol 31:19–22
Leathers TD, Ahlgren JA, Cote GL (1997) Alternansucrase mutants of Leuconostoc mesenteroides strain NRRL B-21138. J Ind Microbiol Biotechnol 18:278–283
Lu J, Perng C, Lee S, Wan C (2000) Use of PCR with universal primers and restriction endonuclease digestions for detection and identification of common bacterial pathogens in cerebrospinal fluid. J Clin Microbiol 38:2076–2080
Misaki A, Torii M, Sawai T, Goldstein IJ (1980) Structure of the dextran of Leuconostoc mesenteroides B-1355. Carbohydr Res 84:273–285
Seymour FR, Knapp RD, Chen ECM, Bishop SH, Jeanes A (1979) Unusual dextrans VIII. Structural analysis of Leuconostoc dextrans containing 3-O-α-d-glucosylated α-d-glucosyl residues in both linear-chain and branch-point positions, or only in branch-point positions, by methylation and by 13C-N.M.R spectroscopy. Carbohyd Res 74:41–62
Slodki ME, England RE, Plattner RD, Dick WE Jr (1986) Methylation analyses of NRRL dextrans by capillary gas–liquid chromatography. Carbohyd Res 156:199–206
Starkey M, Gray KA, Chang SI, Parsek MR (2004) A sticky business: the extracellular polymeric substance matrix of bacterial biofilms. In: Ghannoum M, O’Toole GA (eds) Microbial biofilms. ASM Press, Washington, DC, pp 174–191
Sutherland IW (2001) Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3–9
Takahashi M, Okada S, Uchimura T, Kozaki M (1992) Leuconostoc amelibiosum Schillinger, Holzapfel, and Kandler 1989 is a later subjective synonym of Leuconostoc citreum Farrow, Facklam, and Collins 1989. Int J Syst Bacteriol 42:649–651
Van Cleve JW, Schaefer WC, Rist CE (1956) The structure of NRRL B-512 dextran. Methylation studies. J Am Chem Soc 78:4435–4456
Wozniak DJ, Wyckoff TJO, Starkey M, Keyser R, Azadi P, O’Toole GA, Parsek MR (2003) Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PA01 Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci USA 100:7907–7912
Acknowledgments
The authors thank Melinda S. Nunnally and Eric Hoecker for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and the use of the name by USDA implies no approval of the product to the exclusion of others that may also be suitable.
Rights and permissions
About this article
Cite this article
Leathers, T.D., Bischoff, K.M. Biofilm formation by strains of Leuconostoc citreum and L. mesenteroides . Biotechnol Lett 33, 517–523 (2011). https://doi.org/10.1007/s10529-010-0450-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-010-0450-2