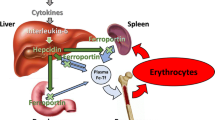
Abstract
Recent studies have revealed that the mammalian immune system directly interferes with siderophore-mediated iron acquisition through siderophore-binding proteins and that the association of certain siderophores, or siderophore modifications, with virulence is a direct response of pathogens to evade these defenses.


Similar content being viewed by others
References
Abergel RJ, Moore EG, Strong RK et al (2006a) Microbial evasion of the immune system: structural modifications of enterobactin impair siderocalin recognition. J Am Chem Soc 128(34):10998–10999. doi:10.1021/ja062476+
Abergel RJ, Wilson MK, Arceneaux JE et al (2006b) Anthrax pathogen evades the mammalian immune system through stealth siderophore production. Proc Natl Acad Sci USA 103(49):18499–18503. doi:10.1073/pnas.0607055103
Abergel RJ, Clifton MC, Pizarro JC et al (2008) The siderocalin/enterobactin interaction: a link between mammalian immunity and bacterial iron transport. J Am Chem Soc 130(34):11524–11534. doi:10.1021/ja803524w
Berger T, Togawa A, Duncan GS et al (2006) Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci USA 103(6):1834–1839. doi:10.1073/pnas.0510847103
Breustedt DA, Korndorfer IP, Redl B et al (2005) The 1.8-a crystal structure of human tear lipocalin reveals an extended branched cavity with capacity for multiple ligands. J Biol Chem 280(1):484–493
Buchanan SK, Smith BS, Venkatramani L et al (1999) Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol 6(1):56–63. doi:10.1038/4931
Budzikiewicz H (2001) Siderophore-antibiotic conjugates used as trojan horses against Pseudomonas aeruginosa. Curr Top Med Chem 1(1):73–82. doi:10.2174/1568026013395524
Carniel E (2001) The Yersinia high-pathogenicity island: an iron-uptake island. Microbes Infect 3(7):561–569. doi:10.1016/S1286-4579(01)01412-5
Crosa JH (1989) Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev 53(4):517–530
Descalzi Cancedda F, Dozin B, Zerega B et al (2000) Ex-FABP: a fatty acid binding lipocalin developmentally regulated in chicken endochondral bone formation and myogenesis. Biochim Biophys Acta 1482(1–2):127–135
Devarajan P (2007) Neutrophil gelatinase-associated lipocalin: new paths for an old shuttle. Cancer Ther 5(B):463–470
Devireddy LR, Teodoro JG, Richard FA et al (2001) Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science 293(5531):829–834. doi:10.1126/science.1061075
Devireddy LR, Gazin C, Zhu X et al (2005) A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 123(7):1293–1305. doi:10.1016/j.cell.2005.10.027
Ellison RT (1994) The effects of lactoferrin on gram-negative bacteria. Adv Exp Med Biol 357:71–90
Fang WK, Xu LY, Lu XF et al (2007) A novel alternative spliced variant of neutrophil gelatinase-associated lipocalin receptor in oesophageal carcinoma cells. Biochem J 403(2):297–303. doi:10.1042/BJ20060836
Fischbach MA, Lin H, Liu DR et al (2004) In vitro characterization of IroB, a pathogen-associated C-glycosyltransferase. Proc Natl Acad Sci USA 102(3):571–576
Fischbach MA, Lin H, Zhou L et al (2006) The pathogen-associated IroA gene cluster mediates bacterial evasion of lipocalin 2. Proc Natl Acad Sci U S A. 103(44):16502–16507
Flo TH, Smith KD, Sato S et al (2004) Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432(7019):917–921. doi:10.1038/nature03104
Flower DR (2000) Beyond the superfamily: the lipocalin receptors. Biochim Biophys Acta 1482(1–2):327–336
Fluckinger M, Haas H, Merschak P et al (2004) Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob Agents Chemother 48(9):3367–3372. doi:10.1128/AAC.48.9.3367-3372.2004
Goetz DH, Willie ST, Armen RS et al (2000) Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochemistry 39(8):1935–1941. doi:10.1021/bi992215v
Goetz DH, Holmes MA, Borregaard N et al (2002) The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell 10(5):1033–1043. doi:10.1016/S1097-2765(02)00708-6
Hartl M, Matt T, Schuler W et al (2003) Cell transformation by the v-myc oncogene abrogates c-Myc/Max-mediated suppression of a C/EBP beta-dependent lipocalin gene. J Mol Biol 333(1):33–46. doi:10.1016/j.jmb.2003.08.018
Holmes MA, Paulsene W, Jide X et al (2005) Siderocalin (Lcn 2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Struct Camb 13(1):29–41
Horwitz LD, Sherman NA, Kong Y et al (1998) Lipophilic siderophores of Mycobacterium tuberculosis prevent cardiac reperfusion injury. Proc Natl Acad Sci USA 95(9):5263–5268. doi:10.1073/pnas.95.9.5263
Hunter HN, Fulton DB, Ganz T et al (2002) The solution structure of human hepcidin, a peptide hormone with antimicrobial activity that is involved in iron uptake and hereditary hemochromatosis. J Biol Chem 277(40):37597–37603. doi:10.1074/jbc.M205305200
Hvidberg V, Jacobsen C, Strong RK et al (2005) The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett 579(3):773–777. doi:10.1016/j.febslet.2004.12.031
Jurado RL (1997) Iron, infections, and anemia of inflammation. Clin Infect Dis 25(4):888–895. doi:10.1086/515549
Kamezaki K, Shimoda K, Numata A et al (2003) The lipocalin 24p3, which is an essential molecule in IL-3 withdrawal-induced apoptosis, is not involved in the G-CSF withdrawal-induced apoptosis. Eur J Haematol 71(6):412–417. doi:10.1046/j.0902-4441.2003.00160.x
Kjeldsen L, Bainton DF, Sengelov H et al (1994) Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 83(3):799–807
Kjeldsen L, Cowland JB, Borregaard N (2000) Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim Biophys Acta 1482(1–2):272–283
Klee SR, Nassif X, Kusecek B et al (2000) Molecular and biological analysis of eight genetic islands that distinguish Neisseria meningitidis from the closely related pathogen Neisseria gonorrhoeae. Infect Immun 68(4):2082–2095. doi:10.1128/IAI.68.4.2082-2095.2000
Lin H, Monaco G, Sun T et al (2005) Bcr-Abl-mediated suppression of normal hematopoiesis in leukemia. Oncogene 24(20):3246–3256. doi:10.1038/sj.onc.1208500
Lovelace LL, Chiswell B, Slade DJ et al (2008) Crystal structure of complement protein C8gamma in complex with a peptide containing the C8gamma binding site on C8alpha: implications for C8gamma ligand binding. Mol Immunol 45(3):750–756. doi:10.1016/j.molimm.2007.06.359
McGrath H Jr, Rigby PG (2004) Hepcidin: inflammation’s iron curtain. Rheumatology (Oxford) 43(11):1323–1325. doi:10.1093/rheumatology/keh345
Meilinger M, Haumer M, Szakmary KA et al (1995) Removal of lactoferrin from plasma is mediated by binding to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor and transport to endosomes. FEBS Lett 360(1):70–74. doi:10.1016/0014-5793(95)00082-K
Moss JE, Cardozo TJ, Zychlinsky A et al (1999) The selC-associated SHI-2 pathogenicity island of Shigella flexneri. Mol Microbiol 33(1):74–83. doi:10.1046/j.1365-2958.1999.01449.x
Muller A, Wilkinson AJ, Wilson KS et al (2006) An [{Fe(mecam)}2]6- bridge in the crystal structure of a ferric enterobactin binding protein. Angew Chem Int Ed Engl 45(31):5132–5136. doi:10.1002/anie.200601198
Neilands JB (1981) Microbial iron compounds. Annu Rev Biochem 50:715–731. doi:10.1146/annurev.bi.50.070181.003435
Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270(45):26723–26726
Nemeth E, Tuttle MS, Powelson J et al (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306(5704):2090–2093. doi:10.1126/science.1104742
Ortlund E, Parker CL, Schreck SF et al (2002) Crystal structure of human complement protein C8gamma at 1.2 A resolution reveals a lipocalin fold and a distinct ligand binding site. Biochemistry 41(22):7030–7037. doi:10.1021/bi025696i
Ratledge C, Dover LG (2000) Iron metabolism in pathogenic bacteria. Annu Rev Microbiol 54:881–941. doi:10.1146/annurev.micro.54.1.881
Raymond KN, Müller G, Matzanke BF (1984) Complexation of iron by siderophores. a review of their solution and structural chemistry and biological function. In: Boschke FL (ed) Topics in current chemistry, vol 123. Springer, Berlin, pp 50–102
Richardson DR (2005) Molecular mechanisms of iron uptake by cells and the use of iron chelators for the treatment of cancer. Curr Med Chem 12(23):2711–2729. doi:10.2174/092986705774462996
Roosenberg JM II, Lin YM, Lu Y et al (2000) Studies and syntheses of siderophores, microbial iron chelators, and analogs as potential drug delivery agents. Curr Med Chem 7(2):159–197
Saito A, Pietromonaco S, Loo AK et al (1994) Complete cloning and sequencing of rat gp330/“megalin”, a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci USA 91(21):9725–9729. doi:10.1073/pnas.91.21.9725
Strausberg RL, Feingold EA, Grouse LH et al (2002) Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci USA 99(26):16899–16903. doi:10.1073/pnas.242603899
Suzuki K, Lareyre JJ, Sanchez D et al (2004) Molecular evolution of epididymal lipocalin genes localized on mouse chromosome 2. Gene 339:49–59. doi:10.1016/j.gene.2004.06.027
Triebel S, Bläser J, Reinke H et al (1992) A 25 kDa a2-microglobulin-related protein is a component of the 125 kDa form of human gelatinase. FEBS Lett 3:386–388. doi:10.1016/0014-5793(92)81511-J
Vokes SA, Reeves SA, Torres AG et al (1999) The aerobactin iron transport system genes in Shigella flexneri are present within a pathogenicity island. Mol Microbiol 33(1):63–73. doi:10.1046/j.1365-2958.1999.01448.x
Warner PJ, Williams PH, Bindereif A et al (1981) ColV plasmid-specific aerobactin synthesis by invasive strains of Escherichia coli. Infect Immun 33(2):540–545
Weinberg ED (1984) Iron withholding: a defense against infection and neoplasia. Physiol Rev 64(1):65–102
Willnow TE, Goldstein JL, Orth K et al (1992) Low density lipoprotein receptor-related protein and gp330 bind similar ligands, including plasminogen activator-inhibitor complexes and lactoferrin, an inhibitor of chylomicron remnant clearance. J Biol Chem 267(36):26172–26180
Winkelmann G (2002) Microbial siderophore-mediated transport. Biochem Soc Trans 30(4):691–696. doi:10.1042/BST0300691
Xu S, Venge P (2000) Lipocalins as biochemical markers of disease. Biochim Biophys Acta 1482(1–2):298–307
Yang J, Goetz D, Li JY et al (2002) An iron delivery pathway mediated by a lipocalin. Mol Cell 10(5):1045–1056. doi:10.1016/S1097-2765(02)00710-4
Yang J, Mori K, Li JY et al (2003) Iron, lipocalin, and kidney epithelia. Am J Physiol Renal Physiol 285(1):F9–F18
Zhou D, Hardt WD, Galan JE (1999) Salmonella typhimurium encodes a putative iron transport system within the centisome 63 pathogenicity island. Infect Immun 67(4):1974–1981
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clifton, M.C., Corrent, C. & Strong, R.K. Siderocalins: siderophore-binding proteins of the innate immune system. Biometals 22, 557–564 (2009). https://doi.org/10.1007/s10534-009-9207-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-009-9207-6