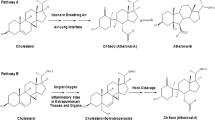
Abstract
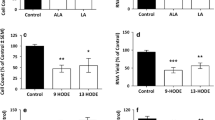
Oxidized low-density lipoproteins play important roles in the development of atherosclerosis and contain several lipid-derived, bioactive molecules which are believed to contribute to atherogenesis. Of these, some cholesterol oxidation products, refered to as oxysterols, are suspected to favor the formation of atherosclerotic plaques involving cytotoxic, pro-oxidant and pro-inflammatory processes. Ten commonly occurring oxysterols (7α-, 7β-hydroxycholesterol, 7-ketocholesterol, 19-hydroxycholesterol, cholesterol-5α,6α-epoxide, cholesterol-5β,6β-epoxide, 22R-, 22S-, 25-, and 27-hydroxycholesterol) were studied for both their cytotoxicity and their ability to induce superoxide anion production (O2⋅ −) and IL-8 secretion in U937 human promonocytic leukemia cells. Cytotoxic effects (phosphatidylserine externalization, loss of mitochondrial potential, increased permeability to propidium iodide, and occurrence of cells with swollen, fragmented and/or condensed nuclei) were only identified with 7β-hydroxycholesterol, 7-ketocholesterol and cholesterol-5β,6β-epoxide, which also induce lysosomal destabilization associated or not associated with the formation of monodansylcadaverine-positive cytoplasmic structures. No relationship between oxysterol-induced cytotoxicity and HMG-CoA reductase activity was found. In addition, the highest O2⋅ − overproduction quantified with hydroethidine was identified with 7β-hydroxycholesterol, 7-ketocholesterol and cholesterol-5β,6β-epoxide, with cholesterol-5α, 6α-epoxide and 25-hydroxycholesterol. The highest capacity to simultaneously stimulate IL-8 secretion (quantified by ELISA and by using a multiplexed, particle-based flow cytometric assay) and enhance IL-8 mRNA levels (determined by RT-PCR) was observed with 7β-hydroxycholesterol and 25-hydroxycholesterol. None of the effects observed for the oxysterols were detected for cholesterol. Therefore, oxysterols may have cytotoxic, oxidative, and/or inflammatory effects, or none whatsoever.
Similar content being viewed by others
Abbreviations
- AO:
-
acridine orange
- MDC:
-
monodansylcadaverine
- HE:
-
hydroethidine
- HMG-CoA:
-
3-hydroxy-3-methylglutaryl-CoA reductase
- PE:
-
phycoerythrin
References
Aupeix K, Weltin D, Mejia JE, et al. Oxysterol-induced apoptosis in human monocytic cell lines. Immunobiol. 1995;194:415–28.
Bansal N, Houle AG, Melnykovych G. Comparison of dexamethasone and lovastatin (mevinolin) as growth inhibitors in cultures of T cell derived human acute leukaemia lines (CEM). Leuk Res. 1989;13:875–82.
Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–27.
Berthier A, Lemaire-Ewing S, Prunet C, et al. Involvement of a calcium-dependent dephosphorylation of BAD associated with the localization of Trpc-1 within lipid rafts in 7-ketocholesterol-induced THP-1 cell apoptosis. Cell Death Differ. 2004;11:897–905.
Beseme F, Astruc ME, Defay R, Crastes de Paulet A. Rat liver cytosol oxysterol-binding protein. FEBS Lett. 1987;210:97–103.
Boisvert WA. Modulation of atherogenesis by chemokines. Trends Cardiovasc Med. 2004;14:161–5.
Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28.
Brown MS, Goldstein JL. Suppression of 3-hydroxy-3-methyl- glutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J Biol Chem. 1974;249:7306–14.
Chen LB. Mitochondrial membrane potential in living cells. Annu Rev Cell Biol. 1988;4:155–81.
Colles SM, Maxson JM, Carlson SG, Chisolm GM. Oxidized LDL-induced injury and apoptosis in atherosclerosis: potential roles for oxysterols. Trends Cardiovasc Med. 2001;11:131–8.
De Nigris F, Lerman A, Ignarro LJ, et al. Oxidation-sensitive mechanisms, vascular apoptosis and atherosclerosis. Trends Mol Med. 2003;9:351–9.
Dzeletovic S, Babiker A, Lund E, Diczfalusy U. Time course of oxy-sterol formation during in vitro oxidation of low density lipoprotein. Chem Phys Lipids. 1995;78:119–28.
Feng B, Yao PM, Li Y, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nature Cell Biol. 2003;5:781–92.
Garcia-Cruset S, Carpenter KL, Guardiola F, Mitchinson MJ. Oxy-sterols in cap and core of human advanced atherosclerotic lesions. Free Radic Res. 1999;30:341–50.
Guicciardi ME, Deussing J, Miyoshi H, et al. Cathepsin B contributes to TNF-α mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127–37.
Guijarro C, Blanco-Colio LM, Ortego M, et al. 3-Hydoxy-3-methylglutaryl coenzyme A reductase and isoprenylation inhibitors induce apoptosis of vascular smooth cells in culture. Circ Res. 1998;83:490–500.
Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–90.
Hwang PLH. High-affinity binding sites for oxygenated sterols in rat liver microsomes: possible identity with anti-estrogen binding sites. Biochim Biophys Acta 1990;1033:154–61.
Ishisaka R, Utsumi T, Kamo T, et al. Participation of a cathepsin L-type protease in the activation of caspase-3. Cell Struct Funct. 1999;24:465–70.
Iuliano L, Micheletta F, Natoli S, et al. Measurement of oxysterols and α-tocopherol in plasma and tissue samples as indices of oxidant stress status. Anal Biochem. 2003;312:217–23.
Janowski BA, Grogan MJ, Jones SA, et al. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc Natl Acad Sci USA. 1999;96:266–71.
Kahn E, Vejux A, Lizard G, et al. Analysis of the fluorescence of monodansyl cadacerine positive cytoplasmic structures during 7-ketocholesterol-induced cell death. Anal Quant Cytol Histol. 2004;27:47–56.
Kandutsch AA, Taylor FR, Shown EP. Different forms of the oxysterol-binding protein. Binding kinetics and stability. J Biol Chem. 1984;259:12388–97.
Kellar KL, Iannone MA. Multiplexed microsphere-based flow cytometric assays. Exp Hematol. 2002;30:1227–37.
Kockx MM, Knaapen MWM. The role of apoptosis in vascular disease. J Pathol. 2001;190:267–80.
Lazier CB, Bapat BV. Antioestrogen binding sites: general and comparative properties. J Steroid Biochem. 1988;31:665–9.
Lemaire S, Lizard G, Monier S, et al. Different patterns of IL-1β secretion, adhesion molecule expression and apoptosis induction in human endothelial cells treated with 7α-,7β-hydroxycholesterol, or 7-ketocholesterol. FEBS Lett. 1998;440:434–9.
Li W, Dalen H, Eaton JW, Yuan XM. Apoptotic death of inflammatory cells in human atheroma. Arterioscler Thromb Vasc Biol. 2001;21:1124–30.
Liu Y, Hulten LM, Wiklund O. Macrophages isolated from human atherosclerotic plaques produce IL-8, and oxysterols may have a regulatory function for IL-8 production. Arterioscler Thromb Vasc Biol. 1997;17:317–23.
Lizard G, Deckert V, Dubrez L, Moisant M, Gambert P, Lagrost L. Induction of apoptosis in endothelial cells treated with cholesterol oxides. Am J Pathol. 1996;148:1625–38.
Lizard G, Fournel S, Genestier L, et al. Kinetics of plasma membrane and mitochondrial alterations in cells undergoing apoptosis. Cytometry. 1995;21:275–83.
Lizard G, Gueldry S, Sordet O, et al. Glutathione is implied in the control of 7-ketocholesterol-induced apoptosis, which is associated with radical oxygen species production. FASEB J. 1998;12:1651–63.
Lizard G, Monier S, Cordelet C, et al. Characterization and comparison of the mode of cell death, apoptosis versus necrosis, induced by 7β-hydroxycholesterol and 7-ketocholesterol in the cells of the vascular wall. Arterioscler Thromb Vasc Biol. 1999;19:1190–200.
Miguet C, Monier S, Bettaieb A, et al. Ceramide generation occurring during 7β-hydroxycholesterol- and 7-ketocholesterol-induced apoptosis is caspase independent and is not required to trigger cell death. Cell Death Differ. 2001;8:83–99.
Miguet-Alfonsi C, Prunet C, Monier S, et al. Analysis of oxidative processes and of myelin figures formation before and after the loss of mitochondrial transmembrane potential during 7β-hydroxycholesterol and 7-ketocholesterol-induced apoptosis: comparison with various pro-apoptotic chemicals. Biochem Pharm. 2002;64:527–41.
Monier S, Samadi M, Prunet C, et al. Impairment of the cytotoxic and oxidative activities of 7β-hydroxycholesterol and 7-ketocholesterol by esterification with oleate. Biochem Biophys Res Commun. 2003;303:814–24.
Moreau M, Brocheriou I, Petit L, Ninio E, Chapman MJ, Rouis M. Interleukin-8 mediates downregulation of tissue inhibitor of metalloproteinase-1 expression in cholesterol-loaded human macrophages: relevance to stability of atherosclerotic plaque. Circulation. 1999;99:420–6.
Morgan E, Varro R, Sepulveda H, et al. Cytometric bead array: multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004;110:252–66.
Mügge A. The role of reactive oxygen species in atherosclerosis. Z Kardiol. 1998;87:851–64.
O’Callaghan JC, Woods JA, O’Brien NM. Comparative study of the cytotoxicity and apoptosis-inducing potential of commonly occurring oxysterols. Cell Biol Toxicol. 2001;17:127–37.
Olkkonen VM. Oxysterol binding protein and its homologues: new regulatory factors involved in lipid metabolism. Curr Opin Lipidol. 2004;15:321–7.
Olsson GM, Rungby J, Rundquist I, Brunk UT. Evaluation of lysosomal stability in living cultured macrophages by cytofluorometry. Effect of silver lactate and hypotonic solutions. Virchows Archiv B Cell Pathol. 1989;56:263–9.
Olsson M, Rundquist I, Brunk U. Flow cytofluorometry of lysosomal acridine orange uptake by living cultured cells. Acta Pathol Microbiol Immunol Scand (Sect A). 1987;95:159–65.
Rosenblat M, Aviram M. Oxysterol-induced activation of macrophage NADPH-oxidase enhances cell-mediated oxidation of LDL in the atherosclerotic apolipoprotein E deficient mouse: inhibitory role for vitamin E. Atherosclerosis. 2002;160:69–80.
Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescin. J Leukoc Biol. 1990;47:440–8.
Salonen JT, Nyyssonen K, Salonen R, et al. Lipoprotein oxidation and progression of atherosclerosis. Circulation. 1997;95:840–5.
Sleer LS, Brown AJ, Stanley KK. Interaction of caveolin with 7-ketocholesterol. Atherosclerosis. 2001;159:49–55.
Smith LL. Cholesterol autoxidation. Chem Phys Lipids. 1987;44:87–125.
Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest. 2002;110:905–11.
Terkeltaub R, Banka CL, Solan J, Santoro D, Brand K, Curtiss LK. Oxidized LDL induces monocytic cell expression of interleukin-8, a chemokine with T-lymphocyte chemotactic activity. Arterioscler Thromb. 1994;14:47–53.
Vaya J, Aviram M, Mahmood S, et al. Selective distribution of oxy-sterols in atherosclerotic lesions and human plasma lipoproteins. Free Radic Res. 2001;34:485–97.
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis: flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39– 51.
Vignali DAA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–55.
Wang N, Tabas I, Winchester R, Ravalli S, Rabbani LE, Tall A. Interleukin 8 is induced by cholesterol loading of macrophages and expressed by macrophage foam cells in human atheroma. J Biol Chem. 1996;15:8837–42.
Witztum JL, Steinberg D. The oxidative modification hypothesis of atherosclerosis: does it hold for humans? Trends Cardiovasc Med. 2001;11:93–102.
Yasunobu Y, Hayashi K, Shingu T, Yamagata T, Kajiyama G, Kambe M. Coronary atherosclerosis and oxidative stress as reflected by autoantibodies against low-density lipoprotein and oxysterols. Atherosclerosis. 2001;155:445–53.
Yeh CG, His B, Faulk WP. Propidium iodide as a nuclear marker in immunofluorescence. II. Use with cellular identification and viability studies. J Immunol Methods. 1981;43:269–75.
Yin J, Chaufour X, McLachlan C, et al. Apoptosis of vascular smooth muscle cells induced by cholesterol and its oxides in vitro and in vivo. Atherosclerosis. 2000;148:365–74.
Yuan XM, Li W, Brunk UT, Dalen H, Chang YH, Sevanian A. Lysosomal dstabilization during macrophage damage induced by cholesterol oxidation products. Free Radic Biol Med. 2000;28:208–18.
Ziedén B, Kaminskas A, Kristenson M, et al. Increased plasma 7β-hydroxycholesterol concentrations in a population with a high risk for cardiovascular disease. Arterioscler Thromb Vasc Biol. 1999;19:967–71.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lemaire-Ewing, S., Prunet, C., Montange, T. et al. Comparison of the cytotoxic, pro-oxidant and pro-inflammatory characteristics of different oxysterols. Cell Biol Toxicol 21, 97–114 (2005). https://doi.org/10.1007/s10565-005-0141-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10565-005-0141-2