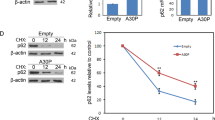
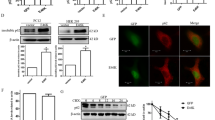
Abstract
Parkinson’s disease (PD) is a chronic progressive neurodegenerative movement disorder characterized by the selective loss of nigrostriatal dopaminergic neurons. However, the molecular pathways leading to the dopaminergic neuron degeneration have remained obscure until recently. Reports demonstrated that reduction of brain-derived neurotrophic factor (BDNF) was involved in the etiology and pathogenesis of PD, but its mechanism has not been elucidated. α-Synuclein has a causal role in Parkinson’s disease, and could interfere with transcriptional regulation of dopamine neurons. In this study, α-synuclein overexpression was found to decrease the expression of BDNF, and also to suppress the transactivation of nuclear factors of activated T-cells (NFAT) and cAMP response element binding protein (CREB), both of which regulate BDNF expression. Furthermore, overexpressed α-synuclein could associate with protein kinase C (PKC) and impair its activity. Meanwhile glycogen synthase kinase-3β (GSK3β) was activated and extracellular signal-regulated protein kinase (ERK) activity was inhibited by overexpression of α-synuclein; both of them were downstream kinases of PKC. Therefore, the impaired PKC signal pathway caused by α-synuclein overexpression might account at least partially for the down-regulation of BDNF.





Similar content being viewed by others
Abbreviations
- BDNF:
-
Brain-derived neurotrophic factor
- NFAT:
-
Nuclear factor of activated T-cells
- MPP+ :
-
1-Methyl, 4-pheny1, pyridinium
- GSK3β:
-
Glycogen synthase kinase 3β
- PD:
-
Parkinson’s disease
- MPTP:
-
1-Methyl, 4-phenyl, 1,2,3,6-tetrahydropyridine
- PKC:
-
Protein kinase C
- CREB:
-
cAMP response element binding protein
- ERK:
-
Extracellular signal-regulated protein kinases
References
Cantuti-Castelvetri I, Keller-McGandy C, Bouzou B, Asteris G, Clark TW, Frosch MP, Standaert DG (2007) Effects of gender on nigral gene expression and Parkinson disease. Neurobiol Dis 26:606–614
Fujita M, Sugama S, Nakai M, Takenouchi T, Wei J, Urano T, Inoue S, Hashimoto M (2007) alpha-Synuclein stimulates differentiation of osteosarcoma cells: relevance to down-regulation of proteasome activity. J Biol Chem 282:5736–5748
Goers J, Manning-Bog AB, McCormack AL, Millett IS, Doniach S, Di Monte DA, Uversky VN, Fink AL (2003) Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry 42:8465–8471
Groth RD, Coicou LG, Mermelstein PG, Seybold VS (2007) Neurotrophin activation of NFAT-dependent transcription contributes to the regulation of pro-nociceptive genes. J Neurochem 102:1162–1174
Grunblatt E, Mandel S, Jacob-Hirsch J, Zeligson S, Amariglo N, Rechavi G, Li J, Ravid R, Roggendorf W, Riederer P, Youdim MB (2004) Gene expression profiling of parkinsonian substantia nigra pars compacta; alterations in ubiquitin-proteasome, heat shock protein, iron and oxidative stress regulated proteins, cell adhesion/cellular matrix and vesicle trafficking genes. J Neural Transm 111:1543–1573
Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, Hughes AJ, Donnan GA (2000) Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol 166:127–135
Iwata A, Miura S, Kanazawa I, Sawada M, Nukina N (2001) alpha-Synuclein forms a complex with transcription factor Elk-1. J Neurochem 77:239–252
Jakes R, Spillantini MG, Goedert M (1994) Identification of two distinct synucleins from human brain. FEBS Lett 345:27–32
Johnson A (2009) TNF-induced activation of pulmonary microvessel endothelial cells: a role for GSK3beta. Am J Physiol Lung Cell Mol Physiol 296:L700–L709
Kaytor MD, Orr HT (2002) The GSK3b signaling cascade and neurodegenerative disease. Curr Opin Neurobiol 12:275–278
Kohno R, Sawada H, Kawamoto Y, Uemura K, Shibasaki H, Shimohama S (2004) BDNF is induced by wild-type alpha-synuclein but not by the two mutants, A30P or A53T, in glioma cell line. Biochem Biophys Res Commun 318:113–118
Kontopoulos E, Parvin JD, Feany MB (2006) a-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum Mol Genet 15:3012–3023
Le W-D, Rowe D, Xie W, Ortiz I, He Y, Appel SH (2001) Microglial activation and dopaminergic cell injury: an in vitro model relevant to Parkinson’s disease. J Neurosci 21:8447–8455
Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Price DL (2002) Human a-synuclein-harboring familial Parkinson’s disease-linked Ala-53 Thr mutation causes neurodegenerative disease with α-synuclein aggregation in transgenic mice. PNAS 99:8968–8973
Leondaritis G, Petrikkos L, Mangoura D (2009) Regulation of the Ras-GTPase activating protein neurofibromin by C-tail phosphorylation: implications for protein kinase C/Ras/extracellular signal-regulated kinase 1/2 pathway signaling and neuronal differentiation. J Neurochem 109:573–583
Maher P (2001) How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J Neurosci 21:2929–2938
Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L (2000) Dopaminergic loss and inclusion body formation in α-synuclein mice: implications for neurodegenerative disorders. Science 287:1265–1267
Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T (1999) Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett 270:45–48
Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B (1999) alpha-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci 19:5782–5791
Parain K, Murer MG, Yan Q, Faucheux B, Agid Y, Hirsch E, Raisman-Vozari R (1999) Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. Neuroreport 10:557–561
Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM (1994) Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol 342:321–334
Siegel GJ, Chauhan NB (2000) Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res Brain Res Rev 33:199–227
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K (2003) a-Synuclein locus triplication causes Parkinson’s disease. Science 302:841
Tian L-L, Zhou Z, Zhang Q, Sun Y-N, Li C-R, Cheng C-H, Zhong Z-Y, Wang S-Q (2007) Protective effect of (±) isoborneol against 6-OHDA-induced apoptosis in SH-SY5Y cells. Cell Physiol Biochem 20:1019–1032
Tomimatsu N, Arakawa Y (2008) Protein kinase C-mediated protection of motoneurons from excitotoxicity. Neurosci Lett 439:143–146
Volakakis N, Malewicz M, Kadkhodai B, Perlmann T, Benoit G (2006) Characterization of the Nurr1 ligand-binding domain co-activator interaction surface. J Mol Endocrinol 37:317–326
Volpicelli F, Caiazzo M, Greco D, Consales C, Leone L, Perrone-Capano C, Colucci D’Amato L, di Porzio U (2007) Bdnf gene is a downstream target of Nurr1 transcription factor in rat midbrain neurons in vitro. J Neurochem 102:441–453
Xu J, Kao SY, Lee FJ, Song W, Yankner BA (2002) Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med 8:600–606
Xu J, Zhong N, Wang H, Elias JE, Kim CY, Woldman I, Pifl C, Gygi SP, Geula C, Yankner BA (2005) The Parkinson’s disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Hum Mol Genet 14:1231–1241
Yacoubian TA, Cantuti-Castelvetri I, Bouzou B, Asteris G, McLean PJ, Hyman BT, Standaert DG (2008) Transcriptional dysregulation in a transgenic model of Parkinson disease. Neurobiol Dis 29:515–528
Yossifoff M, Kisliouk T, Meiri N (2008) Dynamic changes in DNA methylation during thermal control establishment affect CREB binding to the brain-derived neurotrophic factor promoter. Eur J Neurosci 28:2267–2277
Yuan Y, Fu C, Chen H, Wang X, Huang B-R (2006) The Ran binding protein RanBPM interacts with TrkA receptor. Neuro Lett 407:26–31
Yuan Y, Yang B, Zhang W, Hu J, Chen NH (2008) Overexpressed alpha-synuclein regulated the nuclear factor-kappaB signal pathway. Cell Mol Neurobiol 28:21–33
Acknowledgments
The authors thank Professor Perlmann from Ludwig Institute for Cancer Research for kindly donating the NBRE3x constructs. This work was supported by National Key Basic Research & Development Program (973 project Grant No. 2004CB518906), Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT No.IRT0514), the National Natural Science Foundation of China (No. 30572342 and NO. 30271499) and Special Purpose for New Drug Development (2009ZX09303).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, Y., Sun, J., Zhao, M. et al. Overexpression of α-Synuclein Down-Regulates BDNF Expression. Cell Mol Neurobiol 30, 939–946 (2010). https://doi.org/10.1007/s10571-010-9523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-010-9523-y