Abstract
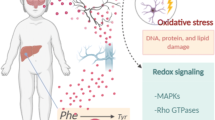
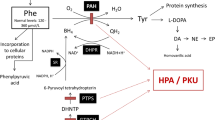
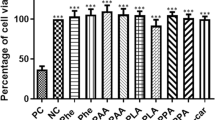
Phenylketonuria (PKU) is an inborn error of amino acid metabolism caused by severe deficiency of phenylalanine hydroxylase activity, leading to the accumulation of phenylalanine and its metabolites in blood and tissues of affected patients. Phenylketonuric patients present as the major clinical feature mental retardation, whose pathomechanisms are poorly understood. In recent years, mounting evidence has emerged indicating that oxidative stress is possibly involved in the pathology of PKU. This article addresses some of the recent developments obtained from animal studies and from phenylketonuric patients indicating that oxidative stress may represent an important element in the pathophysiology of PKU. Several studies have shown that enzymatic and non-enzymatic antioxidant defenses are decreased in plasma and erythrocytes of PKU patients, which may be due to an increased free radical generation or secondary to the deprivation of micronutrients which are essential for these defenses. Indeed, markers of lipid, protein, and DNA oxidative damage have been reported in PKU patients, implying that reactive species production is increased in this disorder. A considerable set of data from in vitro and in vivo animal studies have shown that phenylalanine and/or its metabolites elicit reactive species in brain rodent. These findings point to a disruption of pro-oxidant/antioxidant balance in PKU. Considering that the brain is particularly vulnerable to oxidative attack, it is presumed that the administration of appropriate antioxidants as adjuvant agents, in addition to the usual treatment based on restricted diets or supplementation of tetrahydrobiopterin, may represent another step in the prevention of the neurological damage in PKU.

Similar content being viewed by others
References
Acosta PB, Yannicelli S, Marriage B, Steiner R, Gaffield B, Arnold G, Lewis V, Cho S, Berstein L, Parton P, Leslie N, Korson M (1999) Protein status of infants with phenylketonuria undergoing nutrition management. J Am Coll Nutr 18:102–107
Andersen JK (2004) Oxidative stress in neurodegeneration: cause or consequence? Nat Med 10(Suppl):S18–S25
Araujo GC, Christ SE, Steiner RD, Grange DK, Nardos B, McKinstry RC, White DA (2009) Response monitoring in children with phenylketonuria. Neuropsychology 23:130–134
Artuch R, Vilaseca MA, Moreno J, Lambruschini N, Cambra FJ, Campistol J (1999) Decreased serum ubiquinone-10 concentrations in phenylketonuria. Am J Clin Nutr 70:892–895
Artuch R, Colomé C, Vilaseca MA, Sierra C, Cambra FJ, Lambruschini N, Campistol J (2001) Plasma phenylalanine is associated with decreased serum ubiquinone-10 concentrations in phenylketonuria. J Inherit Metab Dis 24:359–366
Artuch R, Colomé C, Sierra C, Brandi N, Lambruschini N, Campistol J, Ugarte D, Vilaseca MA (2004) A longitudinal study of antioxidant status in phenylketonuric patients. Clin Biochem 37:198–203
Barschak AG, Sitta A, Deon M, de Oliveira MH, Haeser A, Dutra-Filho CS, Wajner M, Vargas CR (2006) Evidence that oxidative stress is increased in plasma from patients with maple syrup urine disease. Metab Brain Dis 21:279–286
Barschak AG, Sitta A, Deon M, Barden AT, Schmitt GO, Dutra-Filho CS, Wajner M, Vargas CR (2007) Erythrocyte glutathione peroxidase activity and plasma selenium concentration are reduced in maple syrup urine disease patients during treatment. Int J Dev Neurosci 25:335–338
Barschak AG, Sitta A, Deon M, Barden AT, Dutra-Filho CS, Wajner M, Vargas CR (2008) Oxidative stress in plasma from maple syrup urine disease patients during treatment. Metab Brain Dis 23:71–80
Bauman ML, Kemper TL (1982) Morphologic and histoanatomic observations of the brain in untreated human phenylketonuria. Acta Neuropathol 58:55–63
Bertelli A, Conte A, Ronca G (1994) L-propionyl carnitine protects erythrocytes and low density lipoproteins against peroxidation. Drugs Exp Clin Res 20:191–197
Bindoli A, Rigobello MP, Deeble DJ (1992) Biochemical and toxicological properties of the oxidation products of catecholamines. Free Radic Biol Med 13:391–405
Bird S, Miller NJ, Collins JE, Rice-Evans CA (1995) Plasma antioxidant capacity in two cases of tyrosinaemia type 1: one case treated with NTBC. J Inherit Metab Dis 18:123–126
Boje KM, Arora PK (1992) Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res 587:250–256
Bridi R, Braun CA, Zorzi GK, Wannmacher CM, Wajner M, Lissi EG, Dutra-Filho CS (2005) Alpha-keto acids accumulating in maple syrup urine disease stimulate lipid peroxidation and reduce antioxidant defences in cerebral cortex from young rats. Metab Brain Dis 20:155–167
Brigelius-Flohé R (1999) Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med 27:951–965
Burri R, Steffen C, Stieger S, Brodbeck U, Colombo JP, Herschkowitz N (1990) Reduced myelinogenesis and recovery in hyperphenylalaninemic rats. Correlation between brain phenylalanine levels, characteristic brain enzymes for myelination, and brain development. Mol Chem Neuropathol 13:57–69
Cadenas E (1997) Basic mechanisms of antioxidant activity. Biofactors 6:391–397
Cadenas E, Sies H (1998) The lag phase. Free Radic Res 28:601–609
Castaño A, Ayala A, Rodríguez-Gómez JA, Herrera AJ, Cano J, Machado A (1997) Low selenium diet increases the dopamine turnover in prefrontal cortex of the rat. Neurochem Int 30:549–555
Castillo M, Zafra MF, Garcia-Peregrin E (1988) Inhibition of brain and liver 3-hydroxy-3-methylglutaryl-CoA reductase and mevalonate-5-pyrophosphate decarboxylase in experimental hyperphenylalaninemia. Neurochem Res 13:551–555
Chinopoulos C, Adam-Vizi V (2001) Mitochondria deficient in complex I activity are depolarized by hydrogen peroxide in nerve terminals: relevance to Parkinson’s disease. J Neurochem 76:302–306
Colomé C, Sierra C, Antònia Vilaseca M (2000) Congenital errors of metabolism: cause of oxidative stress? Med Clin (Barc) 115:111–117
Colomé C, Artuch R, Vilaseca MA, Sierra C, Brandi N, Lambruschini N, Cambra FJ, Campistol J (2003) Lipophilic antioxidants in patients with phenylketonuria. Am J Clin Nutr 77:185–188
Colton C, Wilt S, Gilbert D, Chernyshev O, Snell J, Dubois-Dalcq M (1996) Species differences in the generation of reactive oxygen species by microglia. Mol Chem Neuropathol 28:15–20
Costabeber E, Kessler A, Severo Dutra-Filho C, de Souza Wyse AT, Wajner M, Wannmacher CM (2003) Hyperphenylalaninemia reduces creatine kinase activity in the cerebral cortex of rats. Int J Dev Neurosci 21:111–116
Curtius HC, Niederwieser A, Viscontini M, Leimbacher W, Wegmann H, Blehova B, Rey F, Schaub J, Schmidt H (1981) Serotonin and dopamine synthesis in phenylketonuria. Adv Exp Med Biol 133:277–291
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38
Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A (2006) Biomarkers of oxidative damage in human disease. Clin Chem 52:601–623
Darling G, Mathias P, O’Regan M, Naughten E (1992) Serum selenium levels in individuals on PKU diets. J Inherit Metab Dis 15:769–773
de Freitas MS, de Mattos AG, Camargo MM, Wannmacher CM, Pessoa-Pureur R (1995) Effect of phenylalanine and alpha-methylphenylalanine on in vitro incorporation of 32P into cytoskeletal cerebral proteins. Neurochem Int 26:381–385
de Oliveira Marques F, Hagen ME, Pederzolli CD, Sgaravatti AM, Durigon K, Testa CG, Wannmacher CM, de Souza Wyse AT, Wajner M, Dutra-Filho CS (2003) Glutaric acid induces oxidative stress in brain of young rats. Brain Res 964:153–158
Di Lisa F, Bernardi P (1998) Mitochondrial function as a determinant of recovery or death in cell response to injury. Mol Cell Biochem 184:379–391
Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H (2002) Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med 32:1102–1115
Draper HH, Hadley M (1990) A review of recent studies on the metabolism of exogenous and endogenous malondialdehyde. Xenobiotica 20:901–907
Duez P, Helson M, Some TI, Dubois J, Hanocq M (2000) Chromatographic determination of 8-oxo-7, 8-dihydro-20-deoxyguanosine in cellular DNA: a validation study. Free Radic Res 33:243–260
El Kossi MM, Zakhary MM (2000) Oxidative stress in the context of acute cerebrovascular stroke. Stroke 31:1889–1892
Ellis EM (2007) Reactive carbonyls and oxidative stress: potential for therapeutic intervention. Pharmacol Ther 115:13–24
Ercal N, Aykin-Burns N, Gurer-Orhan H, McDonald JD (2002) Oxidative stress in a phenylketonuria animal model. Free Radic Biol Med 32:906–911
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128
Evans P, Halliwell B (2001) Micronutrients: oxidant/antioxidant status. Br J Nutr 85(suppl 2):S67
Fairbairn DW, Olive PL, O’Neill KL (1995) The comet assay: a comprehensive review. Mutat Res 339:35–59
Fang YZ, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18:872–879
Feksa LR, Cornelio AR, Rech VC, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2002) Alanine prevents the reduction of pyruvate kinase activity in brain cortex of rats subjected to chemically induced hyperphenylalaninemia. Neurochem Res 27:947–952
Fernandes CG, Leipnitz G, Seminotti B, Amaral AU, Zanatta A, Vargas CR, Dutra Filho CS, Wajner M (2010) Experimental evidence that phenylalanine provokes oxidative stress in hippocampus and cerebral cortex of developing rats. Cell Mol Neurobiol 30:317–326
Ferrari R, Ciampalini G, Agnoletti G, Cargnoni A, Ceconi C, Visioli O (1988) Effect of L-carnitine derivatives on heart mitochondrial damage induced by lipid peroxidation. Pharmacol Res Commun 20:125–132
Fontella FU, Pulrolnik V, Gassen E, Wannmacher CM, Klein AB, Wajner M, Dutra-Filho CS (2000) Propionic and L-methylmalonic acids induce oxidative stress in brain of young rats. Neuroreport 11:541–544
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112
Fridovich I (1999) Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann N Y Acad Sci 893:13–18
Gassió R, Artuch R, Vilaseca MA, Fusté E, Colome R, Campistol J (2008) Cognitive functions and the antioxidant system in phenylketonuric patients. Neuropsychology 22:426–431
Giovannini M, Verduci E, Salvatici E, Fiori L, Riva E (2007) Phenylketonuria: dietary and therapeutic challenges. J Inherit Metab Dis 30:145–152
Gülçin I (2006) Antioxidant and antiradical activities of L-carnitine. Life Sci 78:803–811
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Halliwell B, Gutteridge JMC (1999) Oxidative stress: adaptation, damage, repair and death. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine. Oxford University Press, Oxford, pp 246–350
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine. Clarendon Press, Oxford
Hanley WB, Lee AW, Hanley AJ, Lehotay DC, Austin VJ, Schoonheyt WE, Platt BA, Clarke JT (2000) “Hypotyrosinemia” in phenylketonuria. Mol Genet Metab 69:286–294
Hasselbalch S, Knudsen GM, Toft PB, Hogh P, Tedeschi E, Holm S, Videbaek C, Henriksen O, Lou HC, Paulson OB (1996) Cerebral glucose metabolism is decreased in white matter changes in patients with phenylketonuria. Pediatr Res 40:21–24
Hawkins CL, Morgan PE, Davies MJ (2009) Quantification of protein modification by oxidants. Free Radic Biol Med 46:965–988
Heales SJ, Bolaños JP, Brand MP, Clark JB, Land JM (1996) Mitochondrial damage: an important feature in a number of inborn errors of metabolism? J Inherit Metab Dis 19:140–142
Himwich HE (1951) Thought processes as related to brain metabolism in certain abnormal conditions. J Nerv Ment Dis 114:450–458
Ischiropoulos H (2003) Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun 305:776–783
Jenner P, Olanow CW (1996) Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 47:S161–S170
Kaneko T, Tahara S, Matsuo M (1996) Non-linear accumulation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidized DNA damage, during aging. Mutat Res 316:277–285
Kienzle Hagen ME, Pederzolli CD, Sgaravatti AM, Bridi R, Wajner M, Wannmacher CM, Wyse AT, Dutra-Filho CS (2002) Experimental hyperphenylalaninemia provokes oxidative stress in rat brain. Biochim Biophys Acta 1586:344–352
Kovacic P, Pozos RS, Somanathan R, Shangari N, O’Brien PJ (2005) Mechanism of mitochondrial uncouplers, inhibitors, and toxins: focus on electron transfer, free radicals, and structure-activity relationships. Curr Med Chem 12:2601–2623
Krause W, Halminski M, McDonald L, Dembure P, Salvo R, Freides D, Elsas L (1985) Biochemical and neuropsychological effects of elevated plasma phenylalanine in patients with treated phenylketonuria. A model for the study of phenylalanine and brain function in man. J Clin Invest 75:40–48
Lambruschini N, Pérez-Dueñas B, Vilaseca MA, Mas A, Artuch R, Gassió R, Gómez L, Gutiérrez A, Campistol J (2005) Clinical and nutritional evaluation of phenylketonuric patients on tetrahydrobiopterin monotherapy. Mol Genet Metab 86(Suppl 1):S54–S60
Latini A, Borba Rosa R, Scussiato K, Llesuy S, Bello-Klein A, Wajner M (2002) 3-Hydroxyglutaric acid induces oxidative stress and decreases the antioxidant defences in cerebral cortex of young rats. Brain Res 956:367–373
Leipnitz G, Seminotti B, Amaral AU, de Bortoli G, Solano A, Schuck PF, Wyse AT, Wannmacher CM, Latini A, Wajner M (2008) Induction of oxidative stress by the metabolites accumulating in 3-methylglutaconic aciduria in cerebral cortex of young rats. Life Sci 82:652–662
Levine RL (2001) Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med 32:790–796
Lissi E, Salim-Hanna M, Pascual C, del Castillo MD (1995) Evaluation of total antioxidant potential (TRAP) and total antioxidant reactivity from luminol-enhanced chemiluminescence measurements. Free Radic Biol Med 18:153–158
Liu D, Wen J, Liu J, Li L (1999) The roles of free radicals in amyotrophic lateral sclerosis: reactive oxygen species and elevated oxidation of protein, DNA, and membrane phospholipids. FASEB J 13:2318–2328
Lütz Mda G, Feksa LR, Wyse AT, Dutra-Filho CS, Wajner M, Wannmacher CM (2003) Alanine prevents the in vitro inhibition of glycolysis caused by phenylalanine in brain cortex of rats. Metab Brain Dis 18:87–94
Martinez-Cruz F, Pozo D, Osuna C, Espinar A, Marchante C, Guerrero JM (2002) Oxidative stress induced by phenylketonuria in the rat: prevention by melatonin, vitamin E, and vitamin C. J Neurosci Res 69:550–558
Martínez-Cruz F, Osuna C, Guerrero JM (2006) Mitochondrial damage induced by fetal hyperphenylalaninemia in the rat brain and liver: its prevention by melatonin, Vitamin E, and Vitamin C. Neurosci Lett 392:1–4
Masella R, Di Benedetto R, Varì R, Filesi C, Giovannini C (2005) Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem 16:577–586
McCord JM (2000) The evolution of free radicals and oxidative stress. Am J Med 108:652–659
Miller DM, Buettner GR, Aust SD (1990) Transition metals as catalysts of “autoxidation” reactions. Free Radic Biol Med 8:95–108
Moncada S, Higgs A (1993) The l-arginine-nitric oxide pathway. N Engl J Med 329:2002–2012
Moraes TB, Zanin F, da Rosa A, de Oliveira A, Coelho J, Petrillo F, Wajner M, Dutra-Filho CS (2010) Lipoic acid prevents oxidative stress in vitro and in vivo by an acute hyperphenylalaninemia chemically-induced in rat brain. J Neurol Sci 292:89–95
Moyano D, Vilaseca MA, Pineda M, Campistol J, Vernet A, Póo P, Artuch R, Sierra C (1997) Tocopherol in inborn errors of intermediary metabolism. Clin Chim Acta 263:147–155
Nigam MP, Labar DR (1979) The effect of hyperphenylalaninemia on size and density of synapses in rat neocortex. Brain Res 179:195–198
Niki E (2009) Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med 47:469–484
Perry G, Taddeo MA, Petersen RB, Castellani RJ, Harris PL, Siedlak SL, Cash AD, Liu Q, Nunomura A, Atwood CS, Smith MA (2003) Adventiously-bound redox active iron and copper are at the center of oxidative damage in Alzheimer disease. Biometals 16:77–81
Pietz J (1998) Neurological aspects of adult phenylketonuria. Curr Opin Neurol 11:679–688
Poustie VJ, Wildgoose J (2010) Dietary interventions for phenylketonuria. Cochrane Database Syst Rev 20:CD001304
Przyrembel H, Bremer HJ (2000) Nutrition, physical growth, and bone density in treated phenylketonuria. Eur J Pediatr 159(Suppl 2):S129–S135
Rech VC, Feksa LR, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2002) Inhibition of the mitochondrial respiratory chain by phenylalanine in rat cerebral cortex. Neurochem Res 27:353–357
Reilly C, Barrett JE, Patterson CM, Tinggi U, Latham SL, Marrinan A (1990) Trace element nutrition status and dietary intake of children with phenylketonuria. Am J Clin Nutr 52:159–165
Reynolds A, Laurie C, Mosley RL, Gendelman HE (2007) Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol 82:297–325
Reznick AZ, Packer L (1993) Free radicals and antioxidants in muscular neurological diseases and disorders. In: Poli G, Albano E, Dianzani MU (eds) Free radicals: from basic science to medicine. Birkhäuser Verlag, Basel, pp 425–437
Ribas GS, Manfredini V, de Mari JF, Wayhs CY, Vanzin CS, Biancini GB, Sitta A, Deon M, Wajner M, Vargas CR (2010) Reduction of lipid and protein damage in patients with disorders of propionate metabolism under treatment: a possible protective role of L-carnitine supplementation. Int J Dev Neurosci 28:127–132
Richard E, Alvarez-Barrientos A, Pérez B, Desviat LR, Ugarte M (2007) Methylmalonic acidaemia leads to increased production of reactive oxygen species and induction of apoptosis through the mitochondrial/caspase pathway. J Pathol 213:453–461
Richter C (1987) Biophysical consequences of lipid peroxidation in membranes. Chem Phys Lipids 44:175–189
Rodrigues NR, Wannmacher CM, Dutra-Filho CS, Pires RF, Fagan PR, Wajner M (1990) Effect of phenylalanine, p-chlorophenylalanine and alpha-methylphenylalanine on glucose uptake in vitro by the brain of young rats. Biochem Soc Trans 18:419
Rottoli A, Lista G, Zecchini G, Butté C, Longhi R (1985) Plasma selenium levels in treated phenylketonuric patients. J Inherit Metab Dis Suppl 2:127–128
Schulpis KH, Nounopoulos C, Scarpalezou A, Bouloukos A, Missiou-Tsagarakis S (1990) Serum carnitine level in phenylketonuric children under dietary control in Greece. Acta Paediatr Scand 79:930–934
Schulpis KH, Tsakiris S, Karikas GA, Moukas M, Behrakis P (2003) Effect of diet on plasma total antioxidant status in phenylketonuric patients. Eur J Clin Nutr 57:383–387
Schulpis KH, Tsakiris S, Traeger-Synodinos J, Papassotiriou I (2005) Low total antioxidant status is implicated with high 8-hydroxy-2-deoxyguanosine serum concentrations in phenylketonuria. Clin Biochem 38:239–242
Scriver CR, Kaufmann S (2001) The hyperphenylalaninemias. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular basis of inherited disease. McGraw-Hill, New York, pp 1667–1724
Shah SN, Peterson NA, McKean CM (1972) Lipid composition of human cerebral white matter and myelin in phenylketonuria. J Neurochem 19:2369–2376
Sierra C, Vilaseca MA, Moyano D, Brandi N, Campistol J, Lambruschini N, Cambra FJ, Deulofeu R, Mira A (1998) Antioxidant status in hyperphenylalaninemia. Clin Chim Acta 276:1–9
Sies H (1985) Oxidative stress: introductory remarks. In: Sies H (ed) Oxidative stress. Academic Press, London, pp 1–7
Sirtori LR, Dutra-Filho CS, Fitarelli D, Sitta A, Haeser A, Barschak AG, Wajner M, Coelho DM, Llesuy S, Belló-Klein A, Giugliani R, Deon M, Vargas CR (2005) Oxidative stress in patients with phenylketonuria. Biochim Biophys Acta 1740:68–73
Sitta A, Barschak AG, Deon M, Terroso T, Pires R, Giugliani R, Dutra-Filho CS, Wajner M, Vargas CR (2006) Investigation of oxidative stress parameters in treated phenylketonuric patients. Metab Brain Dis 21:287–296
Sitta A, Manfredini V, Biasi L, Treméa R, Schwartz IV, Wajner M, Vargas CR (2009a) Evidence that DNA damage is associated to phenylalanine blood levels in leukocytes from phenylketonuric patients. Mutat Res 679:13–16
Sitta A, Barschak AG, Deon M, de Mari JF, Barden AT, Vanzin CS, Biancini GB, Schwartz IV, Wajner M, Vargas CR (2009b) L-carnitine blood levels and oxidative stress in treated phenylketonuric patients. Cell Mol Neurobiol 29:211–218
Sitta A, Barschak AG, Deon M, Barden AT, Biancini GB, Vargas PR, de Souza CF, Netto C, Wajner M, Vargas CR (2009c) Effect of short- and long-term exposition to high phenylalanine blood levels on oxidative damage in phenylketonuric patients. Int J Dev Neurosci 27:243–247
Smith I, Knowles J (2000) Behaviour in early treated phenylketonuria: a systematic review. Eur J Pediatr 159(Suppl 2):S89–S93
Solano AF, Leipnitz G, De Bortoli GM, Seminotti B, Amaral AU, Fernandes CG, Latini AS, Dutra-Filho CS, Wajner M (2008) Induction of oxidative stress by the metabolites accumulating in isovaleric acidemia in brain cortex of young rats. Free Radic Res 42:707–715
Steiner G, Menzel H, Lombeck I, Ohnesorge FK, Bremer HJ (1982) Plasma glutathione peroxidase after selenium supplementation in patients with reduced selenium state. Eur J Pediatr 138:138–140
Streck EL, Zugno AI, Tagliari B, Franzon R, Wannmacher CM, Wajner M, Wyse AT (2001) Inhibition of rat brain Na+, K+-ATPase activity induced by homocysteine is probably mediated by oxidative stress. Neurochem Res 26:1195–1200
Streck EL, Vieira PS, Wannmacher CM, Dutra-Filho CS, Wajner M, Wyse AT (2003) In vitro effect of homocysteine on some parameters of oxidative stress in rat hippocampus. Metab Brain Dis 18:147–154
Teunissen CE, de Vente J, Steinbusch HW, De Bruijn C (2002) Biochemical markers related to Alzheimer’s dementia in serum and cerebrospinal fluid. Neurobiol Aging 23:485–508
Uchida K (2003) 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res 42:318–343
Ushakova GA, Gubkina HA, Kachur VA, Lepekhin EA (1997) Effect of experimental hyperphenylalaninemia on the postnatal rat brain. Int J Dev Neurosci 15:29–36
Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J (2004) Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266:37–56
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
van Bakel MM, Printzen G, Wermuth B, Wiesmann UN (2000) Antioxidant and thyroid hormone status in selenium-deficient phenylketonuric and hyperphenylalaninemic patients. Am J Clin Nutr 72:976–981
Wajner M, Latini A, Wyse AT, Dutra-Filho CS (2004) The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metab Dis 27:427–448
Weber C, Bysted A, Holmer G (1997) Coenzyme Q10 in the diet-daily intake and relative bioavailability. Mol Aspects Med 18(Suppl):S251–S254
Weglage J, Pietsch M, Fünders B, Koch HG, Ullrich K (1995) Neurological findings in early treated phenylketonuria. Acta Paediatr 84:411–415
Wilke BC, Vidailhet M, Favier A, Guillemin C, Ducros V, Arnaud J, Richard MJ (1992) Selenium, glutathione peroxidase (GSH-Px) and lipid peroxidation products before and after selenium supplementation. Clin Chim Acta 207:137–142
Wyse AT, Wajner M, Brusque A, Wannmacher CM (1995) Alanine reverses the inhibitory effect of phenylalanine and its metabolites on Na+, K(+)-ATPase in synaptic plasma membranes from cerebral cortex of rats. Biochem Soc Trans 23:227S
Wyse AT, Noriler ME, Borges LF, Floriano PJ, Silva CG, Wajner M, Wannmacher CM (1999) Alanine prevents the decrease of Na+, K+-ATPase activity in experimental phenylketonuria. Metab Brain Dis 14:95–101
Wyse AT, Zugno AI, Streck EL, Matté C, Calcagnotto T, Wannmacher CM, Wajner M (2002) Inhibition of Na(+), K(+)-ATPase activity in hippocampus of rats subjected to acute administration of homocysteine is prevented by vitamins E and C treatment. Neurochem Res 27:1685–1689
Zheng M, Storz G (2000) Redox sensing by prokaryotic transcription factors. Biochem Pharmacol 59:1–6
Acknowledgments
This study was supported by CAPES, CNPq, and FIPE/HCPA-Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ribas, G.S., Sitta, A., Wajner, M. et al. Oxidative Stress in Phenylketonuria: What is the Evidence?. Cell Mol Neurobiol 31, 653–662 (2011). https://doi.org/10.1007/s10571-011-9693-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-011-9693-2