Abstract
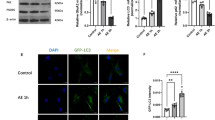
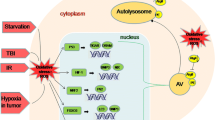
In this study, we investigated the neuroprotective effect of Ro25-6981 against cerebral ischemia/reperfusion injury. Ro25-6981 alone or in combination with rapamycin was intracerebroventricularly administered to rats which suffered transient forebrain ischemia inducing by 4-vessel occlusion and reperfusion. Nissl staining was used to determine the survival of CA1 pyramidal cells of the hippocampus, while immunohistochemistry was performed to measure neuron-specific enolase (NSE) expression. The expression of autophagy-related proteins, such as microtubule-associated protein l light chain 3 (LC3), Beclin 1, and sequestosome 1 (p62), was assessed by immunoblotting. Nissl staining showed that neuronal damage was reduced in the hippocampal CA1 pyramidal layer in rats that received Ro25-6981. The protective effect of Ro25-6981 was dose-dependent, with a significant effect in the middle-dose range. The expression of NSE increased after Ro25-6981 treatment. Ro25-6981 significantly decreased LC3II (which is membrane bound) and Beclin 1, and increased p62. In addition, Ro25-6981 decreased rapamycin-induced neuronal damage and excessive activation of autophagy after I/R. Taken together, the results suggest that Ro25-6981 could suppress ischemic brain injury by regulating autophagy-related proteins during ischemia/reperfusion.





Similar content being viewed by others
References
Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, Itoyama Y (1995) Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke J Cereb Circ 26(8):1478–1489
Abraham RT, Wiederrecht GJ (1996) Immunopharmacology of rapamycin. Annu Rev Immunol 14:483–510. doi:10.1146/annurev.immunol.14.1.483
Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan CY (2006) Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol 169(2):566–583. doi:10.2353/ajpath.2006.051066
Andreasen JT, Bach A, Gynther M, Nasser A, Mogensen J, Stromgaard K, Pickering DS (2013) UCCB01-125, a dimeric inhibitor of PSD-95, reduces inflammatory pain without disrupting cognitive or motor performance: comparison with the NMDA receptor antagonist MK-801. Neuropharmacology 67:193–200. doi:10.1016/j.neuropharm.2012.11.006
Bae N, Chung S, Kim HJ, Cha JW, Oh H, Gu MY, Oh MS, Yang HO (2015) Neuroprotective effect of modified Chungsimyeolda-tang, a traditional Korean herbal formula, via autophagy induction in models of Parkinson’s disease. J Ethnopharmacol 159:93–101. doi:10.1016/j.jep.2014.11.007
Chong ZZ, Li F, Maiese K (2007) The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med 19(2):263–272
Farjam M, Beigi Zarandi FB, Farjadian S, Geramizadeh B, Nikseresht AR, Panjehshahin MR (2014) Inhibition of NR2B-containing N-methyl-d-aspartate receptors (NMDARs) in experimental autoimmune encephalomyelitis, a model of multiple sclerosis. Iranian J Pharm Res 13(2):695–705
Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA (1997) Ro 25-6981, a highly potent and selective blocker of N-methyl-d-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharma Exp Ther 283(3):1285–1292
Gao L, Jiang T, Guo J, Liu Y, Cui G, Gu L, Su L, Zhang Y (2012) Inhibition of autophagy contributes to ischemic postconditioning-induced neuroprotection against focal cerebral ischemia in rats. PLoS One 7(9):e46092. doi:10.1371/journal.pone.0046092
Gogas KR (2006) Glutamate-based therapeutic approaches: NR2B receptor antagonists. Curr Opin Pharmacol 6(1):68–74. doi:10.1016/j.coph.2005.11.001
Guo ZH, Li F, Wang WZ (2009) The mechanisms of brain ischemic insult and potential protective interventions. Neurosci Bull 25(3):139–152. doi:10.1007/s12264-009-0104-3
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441(7095):885–889. doi:10.1038/nature04724
Kirino T (2000) Delayed neuronal death. Neuropathology 20(Suppl):S95–S97
Klionsky DJ, Cuervo AM, Seglen PO (2007) Methods for monitoring autophagy from yeast to human. Autophagy 3(3):181–206
Kocki J, Ulamek-Koziol M, Bogucka-Kocka A, Januszewski S, Jablonski M, Gil-Kulik P, Brzozowska J, Petniak A, Furmaga-Jablonska W, Bogucki J, Czuczwar SJ, Pluta R (2015) Dysregulation of amyloid-beta protein precursor, beta-secretase, presenilin 1 and 2 genes in the rat selectively vulnerable CA1 subfield of hippocampus following transient global brain ischemia. J Alzheimer’s Dis 47(4):1047–1056. doi:10.3233/JAD-150299
Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T (2005) Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169(3):425–434. doi:10.1083/jcb.200412022
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441(7095):880–884. doi:10.1038/nature04723
Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131(6):1149–1163. doi:10.1016/j.cell.2007.10.035
Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N (2004) The role of autophagy during the early neonatal starvation period. Nature 432(7020):1032–1036. doi:10.1038/nature03029
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6(4):463–477
Liu C, Chen S, Kamme F, Hu BR (2005a) Ischemic preconditioning prevents protein aggregation after transient cerebral ischemia. Neuroscience 134(1):69–80. doi:10.1016/j.neuroscience.2005.03.036
Liu CL, Ge P, Zhang F, Hu BR (2005b) Co-translational protein aggregation after transient cerebral ischemia. Neuroscience 134(4):1273–1284. doi:10.1016/j.neuroscience.2005.05.015
Liu P, Cheng H, Roberts TM, Zhao JJ (2009) Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discovery 8(8):627–644. doi:10.1038/nrd2926
Liu H, Qiu H, Xiao Q, Le W (2015) Chronic Hypoxia-Induced Autophagy Aggravates the Neuropathology of Alzheimer’s Disease through AMPK-mTOR Signaling in the APPSwe/PS1dE9 Mouse Model. J Alzheimer’s Dis 48(4):1019–1032. doi:10.3233/JAD-150303
Loschmann PA, De Groote C, Smith L, Wullner U, Fischer G, Kemp JA, Jenner P, Klockgether T (2004) Antiparkinsonian activity of Ro 25-6981, a NR2B subunit specific NMDA receptor antagonist, in animal models of Parkinson’s disease. Exp Neurol 187(1):86–93. doi:10.1016/j.expneurol.2004.01.018
Mackowiak M, Mordalska P, Dudys D, Korostynski M, Bator E, Wedzony K (2011) Cocaine enhances ST8SiaII mRNA expression and neural cell adhesion molecule polysialylation in the rat medial prefrontal cortex. Neuroscience 186:21–31. doi:10.1016/j.neuroscience.2011.04.030
Mizushima N (2010) The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 22(2):132–139. doi:10.1016/j.ceb.2009.12.004
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147(4):728–741. doi:10.1016/j.cell.2011.10.026
Nocjar C, Alex KD, Sonneborn A, Abbas AI, Roth BL, Pehek EA (2015) Serotonin-2C and -2a receptor co-expression on cells in the rat medial prefrontal cortex. Neuroscience 297:22–37. doi:10.1016/j.neuroscience.2015.03.050
Paoletti P, Neyton J (2007) NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol 7(1):39–47. doi:10.1016/j.coph.2006.08.011
Pei DS, Wang XT, Liu Y, Sun YF, Guan QH, Wang W, Yan JZ, Zong YY, Xu TL, Zhang GY (2006) Neuroprotection against ischaemic brain injury by a GluR6-9c peptide containing the TAT protein transduction sequence. Brain J Neurol 129(Pt 2):465–479. doi:10.1093/brain/awh700
Pluta R, Salinska E, Puka M, Stafiej A, Lazarewicz JW (1988) Early changes in extracellular amino acids and calcium concentrations in rabbit hippocampus following complete 15-min cerebral ischemia. Resuscitation 16(3):193–210
Pulsinelli WA, Brierley JB (1979) A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke J Cereb Circ 10(3):267–272
Rami A, Langhagen A, Steiger S (2008) Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy-like cell death. Neurobiol Dis 29(1):132–141. doi:10.1016/j.nbd.2007.08.005
Rebello TJ, Yu Q, Goodfellow NM, Caffrey Cagliostro MK, Teissier A, Morelli E, Demireva EY, Chemiakine A, Rosoklija GB, Dwork AJ, Lambe EK, Gingrich JA, Ansorge MS (2014) Postnatal day 2 to 11 constitutes a 5-HT-sensitive period impacting adult mPFC function. J Soc Neurosci 34(37):12379–12393. doi:10.1523/JNEUROSCI.1020-13.2014
Sadasivan S, Dunn WA Jr, Hayes RL, Wang KK (2008) Changes in autophagy proteins in a rat model of controlled cortical impact induced brain injury. Biochem Biophys Res Commun 373(4):478–481. doi:10.1016/j.bbrc.2008.05.031
Sadasivan S, Zhang Z, Larner SF, Liu MC, Zheng W, Kobeissy FH, Hayes RL, Wang KK (2010) Acute NMDA toxicity in cultured rat cerebellar granule neurons is accompanied by autophagy induction and late onset autophagic cell death phenotype. BMC Neurosci 11:21. doi:10.1186/1471-2202-11-21
Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J (2012) Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther 18(3):250–260. doi:10.1111/j.1755-5949.2012.00295.x
Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306(5698):990–995. doi:10.1126/science.1099993
Smith ML, Auer RN, Siesjo BK (1984) The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathol 64(4):319–332
Ueda M, Nowak TS Jr (2005) Protective preconditioning by transient global ischemia in the rat: components of delayed injury progression and lasting protection distinguished by comparisons of depolarization thresholds for cell loss at long survival times. J Cereb Blood Flow Metab 25(8):949–958. doi:10.1038/sj.jcbfm.9600107
Van Den Bosch L, Van Damme P, Bogaert E, Robberecht W (2006) The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta 1762(11–12):1068–1082. doi:10.1016/j.bbadis.2006.05.002
Wang Y, Han R, Liang ZQ, Wu JC, Zhang XD, Gu ZL, Qin ZH (2008) An autophagic mechanism is involved in apoptotic death of rat striatal neurons induced by the non-N-methyl-d-aspartate receptor agonist kainic acid. Autophagy 4(2):214–226
Wang Y, Wang W, Li D, Li M, Wang P, Wen J, Liang M, Su B, Yin Y (2014) IGF-1 alleviates NMDA-induced excitotoxicity in cultured hippocampal neurons against autophagy via the NR2B/PI3 K-AKT-mTOR pathway. J Cell Physiol 229(11):1618–1629. doi:10.1002/jcp.24607
Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH (2008) Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy 4(6):762–769
Windelborn JA, Lipton P (2008) Lysosomal release of cathepsins causes ischemic damage in the rat hippocampal slice and depends on NMDA-mediated calcium influx, arachidonic acid metabolism, and free radical production. J Neurochem 106(1):56–69. doi:10.1111/j.1471-4159.2008.05349.x
Xie R, Wang P, Cheng M, Sapolsky R, Ji X, Zhao H (2014) Mammalian target of rapamycin cell signaling pathway contributes to the protective effects of ischemic postconditioning against stroke. Stroke 45(9):2769–2776. doi:10.1161/STROKEAHA.114.005406
Xu L, Brink M (2016) mTOR, cardiomyocytes and inflammation in cardiac hypertrophy. Biochim Biophy Acta 7:1894–1903. doi:10.1016/j.bbamcr.2016.01.003 1863
Xu M, Zhang HL (2011) Death and survival of neuronal and astrocytic cells in ischemic brain injury: a role of autophagy. Acta Pharmacol Sin 32(9):1089–1099. doi:10.1038/aps.2011.50
Yin J, Sha S, Chen T, Wang C, Hong J, Jie P, Zhou R, Li L, Sokabe M, Chen L (2015) Sigma-1 (sigma(1)) receptor deficiency reduces beta-amyloid(25-35)-induced hippocampal neuronal cell death and cognitive deficits through suppressing phosphorylation of the NMDA receptor NR2B. Neuropharmacology 89:215–224. doi:10.1016/j.neuropharm.2014.09.027
Zhang L, Shu R, Wang H, Yu Y, Wang C, Yang M, Wang M, Wang G (2014) Hydrogen-rich saline prevents remifentanil-induced hyperalgesia and inhibits MnSOD nitration via regulation of NR2B-containing NMDA receptor in rats. Neuroscience 280:171–180. doi:10.1016/j.neuroscience.2014.09.024
Zheng YQ, Liu JX, Li XZ, Xu L, Xu YG (2009) RNA interference-mediated downregulation of Beclin1 attenuates cerebral ischemic injury in rats. Acta Pharmacol Sin 30(7):919–927. doi:10.1038/aps.2009.79
Acknowledgments
This work was supported by National Natural Science Foundation of China (81271345), the Natural Science Foundation of Jiangsu Higher Education Institutions of China (15KJB180018), and Project of Xuzhou Science and Technology (KC15SH009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Dong, F., Yao, R., Yu, H. et al. Neuroprotection of Ro25-6981 Against Ischemia/Reperfusion-Induced Brain Injury via Inhibition of Autophagy. Cell Mol Neurobiol 37, 743–752 (2017). https://doi.org/10.1007/s10571-016-0409-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-016-0409-5