Abstract
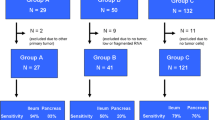
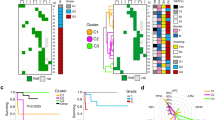
A characteristic of human gastroenteropancreatic neuroendocrine tumors (GEP-NET) is a minute unobtrusive primary tumor which often cannot be detected by common physical examinations. It therefore remains unidentified until the tumor has spread and space-occupying metastases cause clinical symptoms leading to diagnosis. Cases in which the primary cannot be located are referred to as NET with CUP-syndrome (cancer of unknown primary syndrome). With the help of array-CGH (comparative genomic hybridization, Agilent 105K) and gene expression analysis (Agilent 44K), microdissected primaries and their metastases were compared to identify up- and down-regulated genes which can be used as a marker for tumor progression. In a next analysis step, a hierarchical clustering of 41.078 genes revealed three genes [C-type lectin domain family 13 member A (CD302), peptidylprolyl isomerase containing WD40 repeat (PPWD1) and abhydrolase domain containing 14B (ABHD14B)] which expression levels can categorize the metastases into three groups depending on the localization of their primary. Because cancer therapy is dependent on the localization of the primary, the gene expression level of these three genes are promising markers to unravel the CUP syndrome in NET.




Similar content being viewed by others
Abbreviations
- ABHD14B :
-
Abhydrolase domain containing 14B
- CD302 :
-
C-type lectin domain family 13 member A
- CDH22 :
-
Cadherin 22
- DACT2 :
-
Dapper, antagonist of beta-catenin, homolog 2
- CGH:
-
Comparative genomic hybridization
- CUP:
-
Cancer of unknown primary
- GEP-NET:
-
Gastroenteropancreatic neuroendocrine tumor
- GI-NET:
-
Gastrointestinal neuroendocrine tumor
- IHC:
-
Immunohistochemistry
- IL-8:
-
Interleukin 8
- NET:
-
Neuroendocrine tumor
- PDEC:
-
Poorly differentiated carcinoma
- PNET:
-
Pancreatic neuroendocrine tumor
- PPWD1 :
-
Peptidylprolyl isomerase containing WD40 repeat
- RET :
-
Rearranged during transfection (proto-oncogene)
- WDET:
-
Well differentiated neuroendocrine tumor
References
Bornschein J, Kidd M, Malfertheiner P et al (2008) Gastrointestinal neuroendocrine tumors. Dtsch Med Wochenschr 133(28–29):1505–1510
Abbruzzese J, Abbruzzese M, Hess K et al (1994) Unknown primary carcinoma: natural history and prognostic factors in 657 consecutive patients. J Clin Oncol 12(6):1272–1280
Duerr EM, Chung DC (2007) Molecular genetics of neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab 21(1):1–14
Duerr E, Mizukami Y, Ng A et al (2008) Defining molecular classifications and targets in gastroenteropancreatic neuroendocrine tumors through DNA microarray analysis. Endocr Relat Cancer 15(1):243–256
Panzuto F, Nasoni S, Falconi M et al (2005) Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 12(4):1083–1092
Tomasetti P, Campana D, Piscitelli L et al (2005) Endocrine pancreatic tumors: factors correlated with survival. Ann Oncol 16(11):1806–1810
Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182(3):311–322
Rinke A, Müller H, Schade-Brittinger C et al (2009) Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 27(28):4656–4663
Berkovic MC, Jokic M, Marout J et al (2007) IL-6-174 C/G polymorphism in the gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Exp Mol Pathol 83(3):474–479
Capurso G, Lattimore S, Crnogorac-Jurcevic T et al (2006) Gene expression profiles of progressive pancreatic endocrine tumours and their liver metastases reveal potential novel markers and therapeutic targets. Endocr Relat Cancer 13(2):541–558
Hocker M, Wiedenmann B (1998) Molecular mechanisms of enteroendocrine differentiation. Ann N Y Acad Sci 859:160–174
Oberg K (2009) Genetics and molecular pathology of neuroendocrine gastrointestinal and pancreatic tumors (gastroenteropancreatic neuroendocrine tumors). Curr Opin Endocrinol Diabetes Obes 16(1):72–78
Barghorn A, Komminoth P, Bachmann D et al (2001) Deletion at 3p25.3-p23 is frequently encountered in endocrine pancreatic tumours and is associated with metastatic progression. J Pathol 194(4):451–458
Barghorn A, Speel EJ, Farspour B et al (2001) Putative tumor suppressor loci at 6q22 and 6q23–q24 are involved in the malignant progression of sporadic endocrine pancreatic tumors. Am J Pathol 158(6):1903–1911
Guo SS, Arora C, Shimoide AT et al (2002) Frequent deletion of chromosome 3 in malignant sporadic pancreatic endocrine tumors. Mol Cell Endocrinol 190(1–2):109–114
Guo SS, Wu AY, Sawicki MP (2002) Deletion of chromosome 1, but not mutation of MEN-1, predicts prognosis in sporadic pancreatic endocrine tumors. World J Surg 26(7):843–847
Wang EH, Ebrahimi SA, Wu AY et al (1998) Mutation of the MENIN gene in sporadic pancreatic endocrine tumors. Cancer Res 58(19):4417–4420
Zikusoka MN, Kidd M, Eick G et al (2005) The molecular genetics of gastroenteropancreatic neuroendocrine tumors. Cancer 104(11):2292–2309
Melle C, Ernst G, Schimmel B et al (2008) Colon-derived liver metastasis, colorectal carcinoma, and hepatocellular carcinoma can be discriminated by the Ca(2+)-binding proteins S100A6 and S100A11. PLoS One 3(12):e3767
Modlin IM, Oberg K, Chung DC et al (2008) Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 9(1):61–72
Giordano T, Shedden K, Schwartz D et al (2001) Organ-specific molecular classification of primary lung, colon, and ovarian adenocarcinomas using gene expression profiles. Am J Pathol 159(4):1231–1238
Alizadeh A, Ross D, Perou C et al (2001) Towards a novel classification of human malignancies based on gene expression patterns. J Pathol 195(1):41–52
Melle C, Ernst G, Schimmel B et al (2004) A technical triade for proteomic identification and characterization of cancer biomarkers. Cancer Res 64(12):4099–4104
Melle C, Ernst G, Schimmel B et al (2003) Biomarker discovery and identification in laser microdissected head and neck squamous cell carcinoma with ProteinChip technology, two-dimensional gel electrophoresis, tandem mass spectrometry, and immunohistochemistry. Mol Cell Proteomics 2(7):443–452
Ramos-Vara J (2005) Technical aspects of immunohistochemistry. Vet Pathol 42(4):405–426
Bloom G, Yang I, Boulware D et al (2004) Multi-platform, multi-site, microarray-based human tumor classification. Am J Pathol 164(1):9–16
Abbruzzese J, Abbruzzese M, Lenzi R et al (1995) Analysis of a diagnostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol 13(8):2094–2103
Yuhas J, Pazmiño N (1974) Inhibition of subcutaneously growing line 1 carcinomas due to metastatic spread. Cancer Res 34(8):2005–2010
Albertson D, Collins C, McCormick F et al (2003) Chromosome aberrations in solid tumors. Nat Genet 34(4):369–376
Speel E, Richter J, Moch H et al (1999) Genetic differences in endocrine pancreatic tumor subtypes detected by comparative genomic hybridization. Am J Pathol 155(6):1787–1794
Zhao J, Moch H, Scheidweiler A et al (2001) Genomic imbalances in the progression of endocrine pancreatic tumors. Genes Chromosomes Cancer 32(4):364–372
Kytölä S, Höög A, Nord B et al (2001) Comparative genomic hybridization identifies loss of 18q22-qter as an early and specific event in tumorigenesis of midgut carcinoids. Am J Pathol 158(5):1803–1808
Löllgen RM, Hessman O, Szabo E et al (2001) Chromosome 18 deletions are common events in classical midgut carcinoid tumors. Int J Cancer 92(6):812–815
Wang GG, Yao JC, Worah S et al (2005) Comparison of genetic alterations in neuroendocrine tumors: frequent loss of chromosome 18 in ileal carcinoid tumors. Mod Pathol 18(8):1079–1087
Komminoth P, Roth J, Muletta-Feurer S et al (1996) RET proto-oncogene point mutations in sporadic neuroendocrine tumors. J Clin Endocrinol Metab 81(6):2041–2046
Ramaswamy S, Tamayo P, Rifkin R et al (2001) Multiclass cancer diagnosis using tumor gene expression signatures. Proc Natl Acad Sci U S A 98(26):15149–15154
Su A, Welsh J, Sapinoso L et al (2001) Molecular classification of human carcinomas by use of gene expression signatures. Cancer Res 61(20):7388–7393
Yeang C, Ramaswamy S, Tamayo P et al (2001) Molecular classification of multiple tumor types. Bioinformatics 17(Suppl 1):S316–S322
Horlings H, van Laar R, Kerst J et al (2008) Gene expression profiling to identify the histogenetic origin of metastatic adenocarcinomas of unknown primary. J Clin Oncol 26(27):4435–4441
Dennis J, Vass J, Wit E et al (2002) Identification from public data of molecular markers of adenocarcinoma characteristic of the site of origin. Cancer Res 62(21):5999–6005
Shedden K, Taylor J, Enkemann S et al (2008) Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat Med 14(8):822–827
Buckhaults P, Zhang Z, Chen Y et al (2003) Identifying tumor origin using a gene expression-based classification map. Cancer Res 63(14):4144–4149
Tothill R, Kowalczyk A, Rischin D et al (2005) An expression-based site of origin diagnostic method designed for clinical application to cancer of unknown origin. Cancer Res 65(10):4031–4040
Khan J, Wei J, Ringnér M et al (2001) Classification and diagnostic prediction of cancers using gene expression profiling and artificial neural networks. Nat Med 7(6):673–679
Talantov D, Baden J, Jatkoe T et al (2006) A quantitative reverse transcriptase-polymerase chain reaction assay to identify metastatic carcinoma tissue of origin. J Mol Diagn 8(3):320–329
Dumur C, Lyons-Weiler M, Sciulli C et al (2008) Interlaboratory performance of a microarray-based gene expression test to determine tissue of origin in poorly differentiated and undifferentiated cancers. J Mol Diagn 10(1):67–77
Monzon F, Lyons-Weiler M, Buturovic L et al (2009) Multicenter validation of a 1,550-gene expression profile for identification of tumor tissue of origin. J Clin Oncol 27(15):2503–2508
Varadhachary G, Talantov D, Raber M et al (2008) Molecular profiling of carcinoma of unknown primary and correlation with clinical evaluation. J Clin Oncol 26(27):4442–4448
van Laar R, Ma X, de Jong D et al (2009) Implementation of a novel microarray-based diagnostic test for cancer of unknown primary. Int J Cancer 125(6):1390–1397
Bridgewater J, van Laar R, Floore A et al (2008) Gene expression profiling may improve diagnosis in patients with carcinoma of unknown primary. Br J Cancer 98(8):1425–1430
Ma X, Patel R, Wang X et al (2006) Molecular classification of human cancers using a 92-gene real-time quantitative polymerase chain reaction assay. Arch Pathol Lab Med 130(4):465–473
Dennis J, Hvidsten T, Wit E et al (2005) Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res 11(10):3766–3772
Park S, Kim B, Kim J et al (2007) Panels of immunohistochemical markers help determine primary sites of metastatic adenocarcinoma. Arch Pathol Lab Med 131(10):1561–1567
Lubensky I, Zhuang Z (2007) Molecular genetic events in gastrointestinal and pancreatic neuroendocrine tumors. Endocr Pathol 18(3):156–162
Kulke MH, Freed E, Chiang DY et al (2008) High-resolution analysis of genetic alterations in small bowel carcinoid tumors reveals areas of recurrent amplification and loss. Genes Chromosomes Cancer 47(7):591–603
Moll R (2009) The initial CUP situation and CUP syndrome: pathological diagnostics. Pathologe 30(Suppl 2):161–167
von Eggeling F, Ernst G (2007) Microdissected tissue: an underestimated source for biomarker discovery? Biomark Med 1(2):217–219
von Eggeling F, Melle C, Ernst G (2007) Microdissecting the proteome. Proteomics 7(16):2729–2737
Kato M, Khan S, d’Aniello E et al (2007) The novel endocytic and phagocytic C-type lectin receptor DCL-1/CD302 on macrophages is colocalized with F-actin, suggesting a role in cell adhesion and migration. J Immunol 179(9):6052–6063
Kato M, Khan S, Gonzalez N et al (2003) Hodgkin’s lymphoma cell lines express a fusion protein encoded by intergenically spliced mRNA for the multilectin receptor DEC-205 (CD205) and a novel C-type lectin receptor DCL-1. J Biol Chem 278(36):34035–34041
Butler M, Morel A, Jordan W et al (2007) Altered expression and endocytic function of CD205 in human dendritic cells, and detection of a CD205-DCL-1 fusion protein upon dendritic cell maturation. Immunology 120(3):362–371
Davis T, Walker J, Ouyang H et al (2008) The crystal structure of human WD40 repeat-containing peptidylprolyl isomerase (PPWD1). FEBS J 275(9):2283–2295
Disclosure
I have no relevant financial interests related to this manuscript. I certify that all my affiliations with or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript have been disclosed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nicole Posorski, Daniel Kaemmerer are the authors contributed equally.
Merten Hommann, Ferdinand von Eggeling are the authors contributed equally.
Rights and permissions
About this article
Cite this article
Posorski, N., Kaemmerer, D., Ernst, G. et al. Localization of sporadic neuroendocrine tumors by gene expression analysis of their metastases. Clin Exp Metastasis 28, 637–647 (2011). https://doi.org/10.1007/s10585-011-9397-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-011-9397-5