Abstract
Background
“Real-life” data of retention rate and persistence of adalimumab in inflammatory bowel disease are still limited.
Aims
To analyze retention rate, persistence, and safety of adalimumab in a 9-year real-life cohort of inflammatory bowel disease patients.
Methods
In this observational, retrospective single-center study, all adult patients treated with adalimumab as the first- and second-line biological treatment for steroid-dependent or refractory inflammatory bowel disease between March 2008 and March 2017 were included. Primary outcomes were persistence, retention rate, and adverse events; the secondary outcome was the identification of predictors of withdrawal.
Results
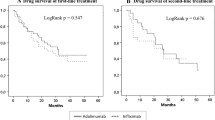
Ninety-six out of 181 patients (53%) withdrew their first course of adalimumab. The retention rate was 47% and 46.9% in Crohn’s disease and ulcerative colitis patients, respectively; median persistence was 26 and 24 months in CD and UC patients, respectively. The cumulative probability of treatment persistence was 80.2%, 54.5%, and 29.6% and 69.6%, 40.4%, and 21.5% in CD and UC patients, respectively. The incidence rate of any adverse event was 12.5/100 patients-year; severe adverse events were 1.7/100 patients-year. The Cox regression revealed that CD patients with baseline disease duration > 72 months have a higher likelihood for withdrawal due to failure and/or adverse events (HR 1.62, 95% CI 1–2.62, p = 0.04); no predictors of discontinuation were found in UC.
Conclusions
Adalimumab showed a great persistence in the first 12 months of therapy and excellent safety profile. Early treatment of CD patients could increase efficacy and reduce the adverse event rate.






Similar content being viewed by others
References
Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333.
Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239.
Colombel J-F, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–265.
Narula N, Kainz S, Petritsch W, et al. The efficacy and safety of either infliximab or adalimumab in 362 patients with anti-TNF-α naïve Crohn’s disease. Aliment Pharmacol Ther. 2016;44:170–180.
Ghosh S, Sandborn WJ, Colombel J-F, et al. Interpreting Registrational Clinical Trials of Biological Therapies in Adults with Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2016;22:2711–2723.
Wolfe F, Michaud K, Dewitt EM. Why results of clinical trials and observational studies of antitumour necrosis factor (anti-TNF) therapy differ: methodological and interpretive issues. Ann Rheum Dis. 2004;63:ii13–ii17.
Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2012;10:1002–1007.
Favalli EG, Pregnolato F, Biggioggero M, et al. Twelve-year retention rate of first-line tumor necrosis factor inhibitors in rheumatoid arthritis: real-life data from a local registry. Arthritis Care Res. 2016;68:432–439.
Sakai R, Tanaka M, Nanki T, et al. Drug retention rates and relevant risk factors for drug discontinuation due to adverse events in rheumatoid arthritis patients receiving anticytokine therapy with different target molecules. Ann Rheum Dis. 2012;71:1820–1826.
Marinas JE, Kim WB, Shahbaz A, Qiang JK, Greaves S, Yeung J. Survival rates of biological therapies for psoriasis treatment in real-world clinical practice: A Canadian multicentre retrospective study. Australas J Dermatol. 2016. https://doi.org/10.1111/ajd.12548.
Leon L, Rodriguez-Rodriguez L, Rosales Z, et al. Long-term drug survival of biological agents in patients with rheumatoid arthritis in clinical practice. Scand J Rheumatol. 2016;45:456–460.
Targownik LE, Tennakoon A, Leung S, et al. Factors associated with discontinuation of anti-TNF inhibitors among persons with IBD: a population-based analysis. Inflamm Bowel Dis. 2017;23:409–420.
Olivera P, Thiriet L, Luc A, Baumann C, Danese S, Peyrin-Biroulet L. Treatment persistence for infliximab versus adalimumab in Crohn’s disease: A 14-year single-center experience. Inflamm Bowel Dis. 2017;23:976–985.
Taxonera C, Iglesias E, Muñoz F, et al. Adalimumab maintenance treatment in ulcerative colitis: outcomes by prior anti-TNF use and efficacy of dose escalation. Dig Dis Sci. 2017;62:481–490. https://doi.org/10.1007/s10620-016-4398-5.
Iborra M, Pérez-Gisbert J, Bosca-Watts MM, et al. Effectiveness of adalimumab for the treatment of ulcerative colitis in clinical practice: comparison between anti-tumour necrosis factor-naïve and non-naïve patients. J Gastroenterol. 2017;52:788–799.
Peyrin-Biroulet L, Salleron J, Filippi J, et al. Anti-TNF monotherapy for Crohn’s Disease: a 13-year multicentre experience. J Crohns Colitis. 2016;10:516–524.
Peters CP, Eshuis EJ, Toxopeüs FM, et al. Adalimumab for Crohn’s disease: long-term sustained benefit in a population-based cohort of 438 patients. J Crohns Colitis. 2014;8:866–875.
Baert F, Glorieus E, Reenaers C, et al. Adalimumab dose escalation and dose de-escalation success rate and predictors in a large national cohort of Crohn’s patients. J Crohns Colitis. 2013;7:154–160.
Allocca M, Bonifacio C, Fiorino G, et al. Efficacy of tumour necrosis factor antagonists in stricturing Crohn’s disease: a tertiary center real-life experience. Dig Liver Dis. 2017;49:872–877.
Orlando A, Renna S, Mocciaro F, et al. Adalimumab in steroid-dependent Crohn’s disease patients: prognostic factors for clinical benefit. Inflamm Bowel Dis. 2012;18:826–831.
Orlando A, Renna S, Mocciaro F, et al. Six year adalimumab efficacy in steroid-dependent Crohn’s disease patients: a prospective single-center real life study. Dig Liver Dis. 2016;48:1314–1317.
Tanaka H, Kamata N, Yamada A, et al. Long-term retention of adalimumab treatment and associated prognostic factors for 1189 patients with Crohn’s disease. J Gastroenterol Hepatol. 2017. https://doi.org/10.1111/jgh.14034.
Italian Group for the Study of Inflammatory Bowel Disease, Armuzzi A, Biancone L, et al. Adalimumab in active ulcerative colitis: a ‘real-life’ observational study. Dig Liver Dis. 2013;45:738–743.
Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25.
Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and Ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649–670.
Travis SPL, Higgins PDR, Orchard T, et al. Review article: defining remission in ulcerative colitis. Aliment Pharmacol Ther. 2011;34:113–124.
Sinha R, Murphy P, Sanders S, et al. Diagnostic accuracy of high-resolution MR enterography in Crohn’s disease: comparison with surgical and pathological specimen. Clin Radiol. 2013;68:917–927.
Schwartz DA, Maltz BE. Treatment of fistulizing inflammatory bowel disease. Gastroenterol Clin North Am. 2009;38:595–610.
Sartini A, Di Girolamo M, Bertani A, Villa E. ‘Blindly’ ADA Dose escalation to 80 mg weekly in Crohn’s disease patients with LOR: is it cost effective or not? Inflamm Bowel Dis. 2015;21:E27–E28.
Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–2789. https://doi.org/10.1016/S0140-6736(17)32641-7.
Pugliese D, Guidi L, Ferraro PM, et al. Paradoxical psoriasis in a large cohort of patients with inflammatory bowel disease receiving treatment with anti-TNF alpha: 5-year follow-up study. Aliment Pharmacol Ther. 2015;42:880–888.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sartini, A., Scaioli, E., Liverani, E. et al. Retention Rate, Persistence and Safety of Adalimumab in Inflammatory Bowel Disease: A Real-Life, 9-Year, Single-Center Experience in Italy. Dig Dis Sci 64, 863–874 (2019). https://doi.org/10.1007/s10620-018-5329-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5329-4