Abstract
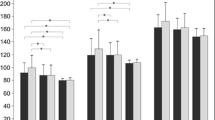
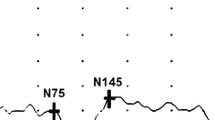
A delay of the peak latency of the pattern visual evoked cortical potentials (pVEP) is accepted as one of the paraclinical evidence for a diagnosis of multiple sclerosis (MS). The purpose of this study was to evaluate the pVEPs in Japanese patients with MS without a history of visual pathway involvement. We studied the medical records of 29 MS patients without any history of visual pathway involvement, and with visual acuity correctable to ≥20/20. The Goldmann visual fields, pupillary light reflexes, and optic disks were normal in all. pVEPs elicited by 3 rev/s (transient) and 12 rev/s (steady-state) were recorded from the MS patients and compared with those recorded from normal subjects. The latency of the P100 component of the transient pVEPs was significantly prolonged in 9/29 (31%) MS patients. A phase lag in the steady-state pVEPs was found in 6/29 (21%) MS patients, and the mean amplitude was significantly smaller. The incidence of cases with abnormal pVEPs is lower than that reported from Europe and United State. This difference is possibly due to racial differences, and the use of different criteria for diagnosing optic neuritis.


Similar content being viewed by others
References
Chiappa KH (1990) Pattern-shift evoked potentials In: Chiappa KH (ed) Evoked potentials in clinical medicine. Raven Press, New York p 111–172
Halliday AM (ed) (1993) Evoked potentials in clinical testing. 2nd edn. Churchill Livingstone, London
Halliday AM, McDonald WI, Mushin J (1973) Visual evoked responses in the diagnosis of multiple sclerosis. Br Med J 4:661–664
Asselman P, Feinsod M, Hoyt WF (1975) Subclinical optic neuropathy in multiple sclerosis. How early VER components reflect axon loss and conduction defects in optic pathways. J Neurol Neurosurg Psychiatry 38:1109–1114
Hoeppner T, Losas F (1978) Visual evoked responses and visual symptoms in multiple sclerosis. J Neurol Neurosurg Psychiatry 41:493–498
Shibasaki H, Kuroiwa Y (1982) Pattern reversal visual evoked potentials in Japanese patient with multiple sclerosis. J Neurol Neurosurg Psychiatry 45:1139–1143
Leys MJJ, Candaele CMLJ, De Rouck AF, Odom JV (1991) Detection of hidden visual loss in multiple sclerosis. Doc Ophthalmol 77:255–264
Pinckers A, Cruysberg JRM (1992) Color vision, visually evoked potentials, and lightness discrimination in patients with multiple sclerosis. Neuro Ophthalmol 12:251–256
Herbst H, Ketabi A, Their P, Dichgans J (1997) Comparison of psychophysical and evoked potential methods in the detection of deficits in multiple sclerosis. Electroencephalogr Clin Neurophysiol 104:82–90
Gronseth GS, Ashman EJ (2000) Practice parameter: the usefulness of evoked potentials in identifying clinically silent lesions in patients with suspected multiple sclerosis (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 54:1720–1725
Davidson AW, Scott RF, Mitchell KW (2004) The effect of contrast reduction on pattern-reversal VEPs in suspected multiple sclerosis and optic neuritis. Doc Ophthalmol 109:157–161
Corallo G, Cicinelli S, Papadia M et al (2005) Conventional perimetry, short-wavelength automated perimetry. Frequency-doubling technology, and visual evoked potentials in the assessment of patients with multiple sclerosis. Eur J Ophthalmol 15:730–738
Acar G, Ozakbas AG, Cakmakci H et al (2004) Visual evoked potential is superior to triple dose magnetic resonance imaging in the diagnosis of optic nerve involvement. Int J Neurosci 114:1025–1033
Poser CM, Paty DW, Scheinberg L et al (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 13:227–231
McDonald WI, Compston A, Edan G et al (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50:121–127
Takasoh M, Adachi-Usami E, Mizota A (1998) Steady-state PVECP is superior to transient PVECP in the diagnosis of optic neuritis. Acta Ophthalmol Scand 76:230–233
Shibasaki H, McDonald WI, Kuroiwa Y (1981) Racial modification of clinical picture of multiple sclerosis: comparison between British and Japanese patients. J Neurol Sci 49:253–271
Alter M, Good J, Okihiro M (1973) Optic neuritis in oriental and Caucasians. Neurology 23:631–639
Hely MA, McManis PG, Walsh JC et al (1986) Visual evoked responses and ophthalmological examination in optic neuritis. J Neurol Sci 75:275–283
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mizota, A., Asaumi, N., Takasoh, M. et al. Pattern visual evoked potentials in Japanese patients with multiple sclerosis without history of visual pathway involvement. Doc Ophthalmol 115, 105–109 (2007). https://doi.org/10.1007/s10633-007-9062-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-007-9062-0