Summary
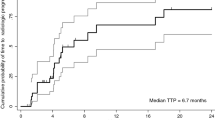
Objectives Gallbladder and cholangiocarcinomas represent a heterogeneous group of malignant diseases that commonly present at an advanced stage and have limited therapeutic options. Based on the role of the Ras-Raf-Mek-Erk pathway and the VEGF axis in biliary carcinomas, we conducted a phase II study of sorafenib in patients with advanced biliary cancers. Methods Eligible patients had no prior therapy for metastatic or unresectable disease. Sorafenib was administered at 400 mg po twice daily continuously. Results The study was terminated after the first stage of accrual due to failure to meet the primary objective. A confirmed response rate of 0% (0%–11%) was observed. Thirty-nine percent of patients demonstrated stable disease (including 2 with unconfirmed PR). PFS was 3 months (95% CI: 2–4 months) and OS 9 months (95% CI: 4–12 months). The most common grade 3 and 4 toxicities included hand-foot skin reaction (13%), bilirubin elevation (13%), venous thromboembolism (10%), AST/ALT elevation (10%) and elevated alkaline phosphatase (10%). Conclusion While treatment with sorafenib did not result in objective responses, patients with biliary cancers receiving this drug had some therapeutic benefit. Additional studies with sorafenib in combination with chemotherapy or other targeted agents may be warranted.


Similar content being viewed by others
References
Cardinale V et al (2010) Intra-hepatic and extra-hepatic cholangiocarcinoma: new insight into epidemiology and risk factors. World J Gastrointest Oncol 2(11):407–16
McGlynn KA, Tarone RE, El-Serag HB (2006) A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev 15(6):1198–203
Hezel AF, Zhu AX (2008) Systemic therapy for biliary tract cancers. Oncologist 13(4):415–23
Choi CW et al (2000) Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am J Clin Oncol 23(4):425–8
Ducreux M et al (2005) A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer 41(3):398–403
Eckel F, Schmid RM (2007) Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 96(6):896–902
Valle J et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362(14):1273–81
Tannapfel A et al (2003) Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut 52(5):706–12
Yoon JH et al (2002) Bile acids inhibit Mcl-1 protein turnover via an epidermal growth factor receptor/Raf-1-dependent mechanism. Cancer Res 62(22):6500–5
Benckert C et al (2003) Transforming growth factor beta 1 stimulates vascular endothelial growth factor gene transcription in human cholangiocellular carcinoma cells. Cancer Res 63(5):1083–92
Yoshikawa D et al (2008) Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer 98(2):418–25
Wilhelm SM et al (2004) BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64(19):7099–109
Green SJ, Dahlberg S (1992) Planned versus attained design in phase II clinical trials. Stat Med 11(7):853–62
Blechacz BR et al (2009) Sorafenib inhibits signal transducer and activator of transcription-3 signaling in cholangiocarcinoma cells by activating the phosphatase shatterproof 2. Hepatology 50(6):1861–70
Sugiyama H et al (2011) Potent in vitro and in vivo antitumor activity of sorafenib against human intrahepatic cholangiocarcinoma cells. J Gastroenterol
Llovet JM et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–90
Iqbal S, Blanke C, Lenz HJ (2006) SWOG S0202: A phase II trial of gemcitabine and capecitabine in patients (pts) with unresectable or metastatic gallbladder cancer or cholangiocarcinoma. Abstract # 4131. in Proceedings of the American Society of Clinical Oncology
Bengala C et al (2010) Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer 102(1):68–72
Yonemoto N et al (2007) A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol 37(11):843–51
Nehls O et al (2008) Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer 98(2):309–15
Harder J et al (2006) Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer 95(7):848–52
Jarnagin WR et al (2006) Differential cell cycle-regulatory protein expression in biliary tract adenocarcinoma: correlation with anatomic site, pathologic variables, and clinical outcome. J Clin Oncol 24(7):1152–60
Acknowledgements
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, NIH, DHHS: CA32102, CA38926, CA20319, CA105409, CA37981, CA16385, CA46441, CA35090, CA35431, CA46282, CA58882, CA45807, CA35176, CA67575, CA45560, CA45808, CA63844, CA12644, CA11083
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Khoueiry, A.B., Rankin, C.J., Ben-Josef, E. et al. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs 30, 1646–1651 (2012). https://doi.org/10.1007/s10637-011-9719-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-011-9719-0