Summary
Until today, there is no systemic treatment available for advanced cholangiocarcinoma (CCA). Recent studies have shown a frequent upregulation of the PI3K-AKT-mTOR and RAF-MEK-ERK pathways in this type of cancer. However, considering their high extend of redundancy and cross-talk, targeting only one pathway is likely to result in therapy failure and emergence of resistances. To provide a rationale for treatment of CCA with inhibitors of these respective pathways, we analyzed the effects of AKT inhibitor MK-2206, MEK inhibitor AZD6244 (ARRY-142886) and mTOR kinase inhibitor AZD8055 on three CCA cell lines in vitro, concerning proliferation, cell signaling and apoptosis. Furthermore, AZD6244 resistant cell lines have been generated to investigate, how their response may be affected by prolonged treatment with only a single inhibitor. Our data demonstrates that co-targeting of both, the PI3K/AKT/mTOR and RAF-MEK-ERK pathway, as well as vertical targeting of AKT and mTOR results in strong synergistic effects on proliferation and cell survival with combination indices below 0.3. Mechanistically, the combinatorial treatment with MK-2206 in addition to AZD8055 is necessary because AKT kinase activity was quickly restored after mTOR kinase inhibition. Interestingly, acquired MEK inhibitor resistance to AZD6244 was reversed by combined treatment with AZD6244 and either MK-2206 or AZD8055. Our data suggest that a combination of inhibitors targeting those respective pathways may be a viable approach for future application in patients with cholangiocarcinoma. Implications: AKT, mTOR and MEK are promising targets for a combinatorial treatment of cholangiocarcinoma cells even after acquisition of MEK inhibitor resistance.





Similar content being viewed by others
Abbreviations
- mTOR:
-
mammalian target of rapamycin
- PI3K:
-
phosphatidylinositol 3-kinase
- PI3KCA:
-
catalytic subunit of phosphatidylinositol 3-kinase
- IRS-1:
-
insulin receptor substrate 1
- BrdU:
-
5-bromo-2’-deoxyuridine
- DMSO:
-
dimethyl sulfoxide
- PBS:
-
phosphate buffered saline
- CCA:
-
cholangiocarcinoma
- CI:
-
combination index
References
Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD (2005) Cholangiocarcinoma. Lancet 366(9493):1303–1314. doi:10.1016/S0140-6736(05)67530-7
Welzel TM, McGlynn KA, Hsing AW, O’Brien TR, Pfeiffer RM (2006) Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst 98(12):873–875. doi:10.1093/jnci/djj234
Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ, Youssef BM, Klimstra D, Blumgart LH (2001) Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 234(4):507–517, discussion 517–509
Jarnagin WR, Shoup M (2004) Surgical management of cholangiocarcinoma. Semin Liver Dis 24(2):189–199. doi:10.1055/s-2004-828895
Yoon JH, Gwak GY, Lee HS, Bronk SF, Werneburg NW, Gores GJ (2004) Enhanced epidermal growth factor receptor activation in human cholangiocarcinoma cells. J Hepatol 41(5):808–814. doi:10.1016/j.jhep.2004.07.016
Wang C, Maass T, Krupp M, Thieringer F, Strand S, Worns MA, Barreiros AP, Galle PR, Teufel A (2009) A systems biology perspective on cholangiocellular carcinoma development: focus on MAPK-signaling and the extracellular environment. J Hepatol 50(6):1122–1131. doi:10.1016/j.jhep.2009.01.024
Schmitz KJ, Lang H, Wohlschlaeger J, Sotiropoulos GC, Reis H, Schmid KW, Baba HA (2007) AKT and ERK1/2 signaling in intrahepatic cholangiocarcinoma. World J Gastroenterol 13(48):6470–6477
Hezel AF, Deshpande V, Zhu AX (2010) Genetics of biliary tract cancers and emerging targeted therapies. J Clin Oncol 28(21):3531–3540. doi:10.1200/JCO.2009.27.4787
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA (2007) Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 1773(8):1263–1284. doi:10.1016/j.bbamcr.2006.10.001
Davies BR, Logie A, McKay JS, Martin P, Steele S, Jenkins R, Cockerill M, Cartlidge S, Smith PD (2007) AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther 6(8):2209–2219. doi:10.1158/1535-7163.MCT-07-0231
Bekaii-Saab T, Phelps MA, Li X, Saji M, Goff L, Kauh JS, O’Neil BH, Balsom S, Balint C, Liersemann R, Vasko VV, Bloomston M, Marsh W, Doyle LA, Ellison G, Grever M, Ringel MD, Villalona-Calero MA (2011) Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 29(17):2357–2363. doi:10.1200/JCO.2010.33.9473
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149(2):274–293. doi:10.1016/j.cell.2012.03.017
Engelman JA (2009) Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9(8):550–562. doi:10.1038/nrc2664
Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H (2010) MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther 9(7):1956–1967. doi:10.1158/1535-7163.MCT-09-1012
Inoki K, Corradetti MN, Guan KL (2005) Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet 37(1):19–24. doi:10.1038/ng1494
Sparks CA, Guertin DA (2010) Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene 29(26):3733–3744. doi:10.1038/onc.2010.139
Liu P, Gan W, Inuzuka H, Lazorchak AS, Gao D, Arojo O, Liu D, Wan L, Zhai B, Yu Y, Yuan M, Kim BM, Shaik S, Menon S, Gygi SP, Lee TH, Asara JM, Manning BD, Blenis J, Su B, Wei W (2013) Sin1 phosphorylation impairs mTORC2 complex integrity and inhibits downstream Akt signalling to suppress tumorigenesis. Nat Cell Biol 15(11):1340–1350. doi:10.1038/ncb2860
Wang Z, Zheng T, Wu Q, Wang J, Wu C (2012) Immunohistochemical analysis of the mTOR pathway in intrahepatic cholangiocarcinoma. Neoplasma 59(2):137–141
Chung JY, Hong SM, Choi BY, Cho H, Yu E, Hewitt SM (2009) The expression of phospho-AKT, phospho-mTOR, and PTEN in extrahepatic cholangiocarcinoma. Clin Cancer Res 15(2):660–667. doi:10.1158/1078-0432.CCR-08-1084
Markman B, Dienstmann R, Tabernero J (2010) Targeting the PI3K/Akt/mTOR pathway--beyond rapalogs. Oncotarget 1 (7):530–543. doi:101012 [pii]
Lv X, Ma X, Hu Y (2013) Furthering the design and the discovery of small molecule ATP-competitive mTOR inhibitors as an effective cancer treatment. Expert Opin Drug Discov. doi:10.1517/17460441.2013.800479
Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, Baselga J, Guichard S, Rosen N (2011) mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov 1(3):248–259. doi:10.1158/2159-8290.CD-11-0085
Pal SK, Reckamp K, Yu H, Figlin RA (2010) Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs 19(11):1355–1366. doi:10.1517/13543784.2010.520701
Mendoza MC, Er EE, Blenis J (2011) The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36(6):320–328. doi:10.1016/j.tibs.2011.03.006
Meng J, Dai B, Fang B, Bekele BN, Bornmann WG, Sun D, Peng Z, Herbst RS, Papadimitrakopoulou V, Minna JD, Peyton M, Roth JA (2010) Combination treatment with MEK and AKT inhibitors is more effective than each drug alone in human non-small cell lung cancer in vitro and in vivo. PLoS One 5(11):e14124. doi:10.1371/journal.pone.0014124
Scherdin G, Garbrecht M, Klouche M (1987) In vitro interaction of á-difluoromethylornithine (DFMO) and human recombinant interferon-α (rIFN-α) on human cancer cell lines. Immunobiology 175(1–2):1–143
Saijyo S, Kudo T, Suzuki M, Katayose Y, Shinoda M, Muto T, Fukuhara K, Suzuki T, Matsuno S (1995) Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med 177(1):61–71
Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D, Prellwitz W, Buschenfelde KH M z (1985) Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol 1(6):579–596
Grabinski N, Bartkowiak K, Grupp K, Brandt B, Pantel K, Jucker M Distinct functional roles of Akt isoforms for proliferation, survival, migration and EGF-mediated signalling in lung cancer derived disseminated tumor cells. Cell Signal 23 (12):1952–1960. doi:S0898-6568 (11) 00200-2 [pii] 10.1016/j.cellsig.2011.07.003
Ewald F, Grabinski N, Grottke A, Windhorst S, Norz D, Carstensen L, Staufer K, Hofmann BT, Diehl F, David K, Schumacher U, Nashan B, Jucker M (2013) Combined targeting of AKT and mTOR using MK-2206 and RAD001 is synergistic in the treatment of cholangiocarcinoma. Int J Cancer. doi:10.1002/ijc.28214
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Kandil E, Tsumagari K, Ma J, Abd Elmageed ZY, Li X, Slakey D, Mondal D, Abdel-Mageed AB (2013) Synergistic inhibition of thyroid cancer by suppressing MAPK/PI3K/AKT pathways. J Surg Res 184 (2):898–906. doi:S0022-4804 (13) 00246-1 [pii] 10.1016/j.jss.2013.03.052
Liao Y, Hung MC (2010) Physiological regulation of Akt activity and stability. Am J Transl Res 2(1):19–42
Fritsche-Guenther R, Witzel F, Sieber A, Herr R, Schmidt N, Braun S, Brummer T, Sers C, Bluthgen N (2011) Strong negative feedback from Erk to Raf confers robustness to MAPK signalling. Mol Syst Biol 7:489. doi:msb201127 [pii] 10.1038/msb.2011.27
Yamanaka K, Petrulionis M, Lin S, Gao C, Galli U, Richter S, Winkler S, Houben P, Schultze D, Hatano E, Schemmer P (2013) Therapeutic potential and adverse events of everolimus for treatment of hepatocellular carcinoma - systematic review and meta-analysis. Cancer Med 2(6):862–871. doi:10.1002/cam4.150
Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y, Chirieac LR, Kaur R, Lightbown A, Simendinger J, Li T, Padera RF, Garcia-Echeverria C, Weissleder R, Mahmood U, Cantley LC, Wong KK (2008) Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 14(12):1351–1356. doi:10.1038/nm.1890
Grabinski N, Ewald F, Hofmann BT, Staufer K, Schumacher U, Nashan B, Jucker M (2012) Combined targeting of AKT and mTOR synergistically inhibits proliferation of hepatocellular carcinoma cells. Mol Cancer 11:85. doi:1476-4598-11-85 [pii] 10.1186/1476-4598-11-85
Meng J, Fang B, Liao Y, Chresta CM, Smith PD, Roth JA (2010) Apoptosis induction by MEK inhibition in human lung cancer cells is mediated by Bim. PLoS One 5(9):e13026. doi:10.1371/journal.pone.0013026
Renshaw J, Taylor KR, Bishop R, Valenti M, De Haven Brandon A, Gowan S, Eccles SA, Ruddle R, Johnson LD, Raynaud FI, Selfe J, Thway K, Pietsch T, Pearson AD, Shipley J (2013) Dual blockade of the PI3K/AKT/mTOR (AZD8055) and RAS/MEK/ERK (AZD6244) pathways synergistically inhibits rhabdomyosarcoma cell growth in vitro and in vivo. Clin Cancer Res. doi:10.1158/1078-0432.CCR-13-0850
Ho AL, Musi E, Ambrosini G, Nair JS, Deraje Vasudeva S, de Stanchina E, Schwartz GK (2012) Impact of combined mTOR and MEK inhibition in uveal melanoma is driven by tumor genotype. PLoS One 7 (7):e40439. doi:10.1371/journal.pone.0040439 PONE-D-12-04817 [pii]
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307 (5712):1098–1101. doi:307/5712/1098 [pii] 10.1126/science.1106148
Little AS, Balmanno K, Sale MJ, Newman S, Dry JR, Hampson M, Edwards PA, Smith PD, Cook SJ (2011) Amplification of the driving oncogene, KRAS or BRAF, underpins acquired resistance to MEK1/2 inhibitors in colorectal cancer cells. Sci Signal 4 (166):ra17. doi:4/166/ra17 [pii] 10.1126/scisignal.2001752
Corcoran RB, Dias-Santagata D, Bergethon K, Iafrate AJ, Settleman J, Engelman JA (2010) BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci Signal 3 (149):ra84. doi:3/149/ra84 [pii] 10.1126/scisignal.2001148
44. Atefi M, von Euw E, Attar N, Ng C, Chu C, Guo D, Nazarian R, Chmielowski B, Glaspy JA, Comin-Anduix B, Mischel PS, Lo RS, Ribas A (2011) Reversing melanoma cross-resistance to BRAF and MEK inhibitors by co-targeting the AKT/mTOR pathway. PLoS One 6 (12):e28973. doi:10.1371/journal.pone.0028973 PONE-D-11-08524 [pii]
Turke AB, Song Y, Costa C, Cook R, Arteaga CL, Asara JM, Engelman JA (2012) MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors. Cancer Res 72(13):3228–3237. doi:10.1158/0008-5472.CAN-11-3747
Meng J, Peng H, Dai B, Guo W, Wang L, Ji L, Minna JD, Chresta CM, Smith PD, Fang B, Roth JA (2009) High level of AKT activity is associated with resistance to MEK inhibitor AZD6244 (ARRY-142886). Cancer Biol Ther 8(21):2073–2080
Acknowledgements
We are grateful to Dr. Knuth from the University Hospital Zürich, Department of Oncology for providing SK-ChA-1 cells. Florian Ewald is a fellow of the University Cancer Center Hamburg (UCCH).
Conflict of interest statement
There is no conflict of interest.
Authors’ contributions
FE, DN, BN and MJ designed the study. FE, DN, AG and BH performed the experiments and interpreted the experimental findings. FE, DN and MJ drafted the manuscript and wrote the final version of the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Florian Ewald and Dominik Nörz contributed equally to this work
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
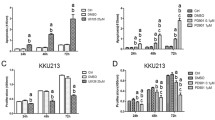
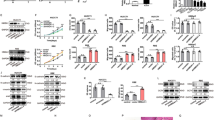
Cells were treated with the indicated concentrations of AZD6244 or AZD8055 for 24h. PI3K-AKT-mTOR and RAF-MEK-ERK signaling pathway activity was analyzed by western blot with antibodies directed against the indicated targets. HSC70 served as loading control. (PDF 246 kb)
Online Resource 2
CCA cell lines were treated with increasing concentrations of AZD8055 (a) or AZD6244 (b), controls were treated with DMSO. Proliferation of CCA cell lines was analyzed after 72h by BrdU incorporation. Each data point represents mean of three independent experiments, normalized to controls. The respective concentrations of AZD6244 and AZD8055 resulting in 50% inhibition of proliferation are given in (c). (PDF 311 kb)
Online Resource 3
Fractional Effect blots were computed based on the experiment as described in Fig. 1. CI values from 0.3 to 0.7 are considered to indicate synergism, and CI values below 0.3 are considered to represent strong synergism. (PDF 138 kb)
Rights and permissions
About this article
Cite this article
Ewald, F., Nörz, D., Grottke, A. et al. Dual Inhibition of PI3K-AKT-mTOR- and RAF-MEK-ERK-signaling is synergistic in cholangiocarcinoma and reverses acquired resistance to MEK-inhibitors. Invest New Drugs 32, 1144–1154 (2014). https://doi.org/10.1007/s10637-014-0149-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0149-7