Abstract
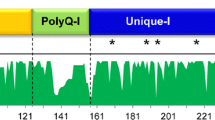
Many of the unique properties of wheat flour are derived from seed storage proteins such as the α-gliadins. In this study these α-gliadin genes from diploid Triticeae species were systemically characterized, and divided into 3 classes according to the distinct organization of their protein domains. Our analyses indicated that these α-gliadins varied in the number of cysteine residues they contained. Most of the α-gliadin genes were grouped according to their genomic origins within the phylogenetic tree. As expected, sequence alignments suggested that the repetitive domain and the two polyglutamine regions were responsible for length variations of α-gliadins as were the insertion/deletion of structural domains within the three different classes (I, II, and III) of α-gliadins. A screening of celiac disease toxic epitopes indicated that the α-gliadins of the class II, derived from the Ns genome, contain no epitope, and that some other genomes contain much fewer epitopes than the A, S(B) and D genomes of wheat. Our results suggest that the observed genetic differences in α-gliadins of Triticeae might indicate their use as a fertile ground for the breeding of less CD-toxic wheat varieties.



Similar content being viewed by others
References
Anderson OD, Greene FC (1997) The α-gliadin gene family. II. DNA and protein sequence variation, subfamily structure, and origins of pseudogenes. Theor Appl Genet 95:59–65. doi:10.1007/s001220050532
Anderson RP, Degano P, Godkin AJ, Jewell DP, Hill AVS (2000) In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat Med 6:337–342. doi:10.1038/73200
Anderson OD, Hisa CC, Torres V (2001) The wheat γ-gliadin genes: characterization of ten new sequences and further understanding of γ-gliadin gene family structure. Theor Appl Genet 103:323–330. doi:10.1007/s00122-001-0551-3
Arentz-Hansen H, Körner R, Molberg O, Quarsten H, Vader W, Kooy YMC, Lundin KEA, Koning F, Roepstorff P, Sollid LM, McAdam SN (2000) The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J Exp Med 191:603–612. doi:10.1084/jem.191.4.603
Arentz-Hansen H, McAdam SN, Molberg O, Fleckenstein B, Lundin KEA, Jorgensen TJD, Jung G, Roepstorff P, Sollid LM (2002) Celiac lesion T cells recognize epitopes that cluster in regions of gliadins rich in proline residues. Gastroenterology 123:803–809. doi:10.1053/gast.2002.35381
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure from small quantities of fresh leaf tissues. Phytochem Bull 19:11–15
Gianfrani C, Troncone R, Mugione P, Cosentini E, De Pascale M, Faruolo C, Senger S, Terrazzano G, Southwood S, Auricchio S, Sette A (2003) Celiac disease association with CD8+ T cell responses: identification of a novel gliadin-derived HLA-A2-restricted epitope. J Immunol 170:2719–2726
Gustafson JP, Butler E, Mcintyre CL (1990) Physical mapping of a low-copy DNA sequence in rye (Secale cereale L.). Proc Natl Sci USA 87:1899–1902
Koning F, Gilissen L, Wijmenga C (2005) Gluten: a two edged sword. Immunopathogenesis of celiac disease. Springer Semin Immun 27:217–232. doi:10.1007/s00281-005-0203-9
Li GR, Ren ZL, Liu C, Zhou JP, Yang ZJ (2008) Isolation and sequence analysis of α-gliadin genes from Dasypyrum breviaristatum. Acta Agron Sin 34:1097–1103. doi:10.3724/SP.J.1006.2008.01097
Li G, Zhang T, Wei P, Jia J, Yang Z (2010a) Sequence analysis of α-gliadin genes from Aegilops tauschii native to China. Asian J Agric Sci 2:128–135
Li G, Zhang T, Ban Y, Yang Z (2010b) Molecular characterization and evolutionary analysis of α-gliadin genes from Eremopyrum bonaepartis (Triticeae). J Agric Sci 2:30–36
Lundin KEA, Scott H, Hansen T, Paulsen G, Halstensen TS, Fausa O, Thorsby E, Sollid LM (1993) Gliadin-specific, Hla-Dq (α1*0501, β1*0201) restricted T-cells isolated from the small-intestinal mucosa of celiac disease patients. J Exp Med 178:187–196. doi:10.1084/jem.178.1.187
Ma ZC, Wei YM, Yan ZH, Zheng YL (2007) Characterization of α-gliadin genes from diploid wheats and the comparative analysis with those from polyploid wheats. Russ J Genet 43:1286–1293. doi:10.1134/S1022795407110117
Maiuri L, Troncone R, Mayer M, Coletta S, Picarelli A, De Vincenzi M, Pavone V, Auricchio S (1996) In vitro activities of A-gliadin related synthetic peptides: damaging effect on the atrophic coeliac mucosa and activation of mucosal immune response in the treated coeliac mucosa. Scand J Gastroenterol 31:247–253
Mantzaris G, Jewell DP (1991) In vivo toxicity of a synthetic dodecapeptide from α-gliadin in patients with celiac-disease. Scand J Gastroenterol 26:392–398
Martucci S, Fraser JS, Biagi F, Corazza GR, Ciclitira PJ, Ellis HJ (2003) Characterizing one of the DQ2 candidate epitopes in coeliac disease: a-gliadin 51-70 toxicity assessed using an organ culture system. Eur J Gastroen Hepat 15:1293–1298
Mazzarella G, Maglio M, Paparo F, Nardone G, Stefanile R, Greco L, van de Wal Y, Kooy Y, Koning F, Auricchio S, Troncone R (2003) An immunodominant DQ8 restricted gliadin peptide activates small intestinal immune response in in vitro cultured mucosa from HLA-DQ8 positive but not HLA-DQ8 negative coeliac patients. Gut 52:57–62. doi:10.1136/gut.52.1.57
McManus R, Kelleher D (2003) Celiac disease-the villain unmasked? New Engl J Med 348:2573–2574
Molberg Ø, Uhlen AK, Jensen T, Flæte NS, Fleckenstein B, Arentz-Hansen H, Raki M, Lundin KEA, Sollid LM (2005) Mapping of gluten T cell epitopes in the bread wheat ancestors: implications for celiac disease. Gastroenterology 128:393–401. doi:10.1053/j.gastro.2004.11.003
Müller S, Wieser H (1995) The location of disulphide bonds in α-type gliadins. J Cereal Sci 22:21–27. doi:10.1016/S0733-5210(05)80004-9
Payne PI (1987) Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Annu Rev Plant Physiol 38:141–153
Payne PI, Holt LM, Jackson EA, Law CN (1984) Wheat storage proteins: their genetics and potential for manipulation by plant breeding. Philos Trans R Soc Lond Ser B 304:359–371. doi:10.1098/rstb.1984.0031
Payne PI, Nightinglale MA, Krattiger AF, Holt LM (1987) The relationship between HMW glutenin subunit composition and the bread-making quality of British-grown wheat varieties. J Sci Food Agric 40:51–65. doi:10.1002/jsfa.2740400108
Qi PF, Wei YM, Yue YW, Yan ZH, Zheng YL (2006) Biochemical and molecular characterization of gliadins. Mol Biol 40:713–723. doi:10.1134/S0026893306050050
Qi PF, Wei YM, Ouellet T, Chen Q, Tan X, Zheng YL (2009) The γ-gliadin multigene family in common wheat (Triticum aestivum) and its closely related species. BMC genomics 10:168. doi:10.1186/1471-2164-10-168
Qi PF, Wei YM, Chen Q, Ouellet T, Ai J, Chen GY, Li W, Zheng YL (2011) Identification of novel α-gliadin genes. Genome 54:244–252. doi:10.1139/G10-114
Qi PF, Wei YM, Chen GY, Jiang QT, Liu YX, Li W, Zheng YL (2012) Development of chromosome 6D-specific markers for α-gliadin genes and their use in assessing dynamic changes at the Gli-2 loci. Mol Breed 29:199–208. doi:10.1007/s11032-010-9539-5
Reeves CD, Okita TW (1987) Analysis of α/β-type gliadin genes from diploid and hexaploid wheats. Gene 52:257–266. doi:10.1016/0378-1119(87)90052-7
Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X (2009) DOG1.0: illustrator of protein domain structures. Cell Res 19:271–273. doi:10.1038/cr.2009.6
Shan L, Molberg ø, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C (2002) Structural basis for gluten intolerance in celiac disease. Science 297:2275–2279. doi:10.1126/science.1074129
Shewry PR, Halford NG (2002) Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot 53:947–958. doi:10.1093/jexbot/53.370.947
Shewry PR, Tatham AS (1990) The prolamin storage proteins of cereal seeds: structure and evolution. Biochem J 267:1–12
Shewry PR, Napier JA, Tatham AS (1995) Seed storage proteins: structures and biosynthesis. Plant Cell 7:945–956. doi:10.1105/tpc.7.7.945
Spaenij-Dekking L, Kooy-Winkelaar Y, Van Veelen P, Drijfhout JW, Jonker H, Van Soest L, Smulders MJM, Bosch D, Gilissen L, Koning F (2005) Natural variation in toxicity of wheat: potential for selection of nontoxic varieties for celiac disease patients. Gastroenterology 129:797–806. doi:10.1053/j.gastro.2005.06.017
Stern M, Ciclitira PJ, van Eckert R, Feighery C, Janssen FW, Méndez E, Mothes T, Troncone R, Wieser H (2001) Analysis and clinical effects of gluten in coeliac disease. Eur J Gastroen Hepat 13:741–747
Sturgess R, Day P, Ellis HJ, Lundin KEA, Gjertsen HA, Kontakou M, Ciclitira PJ (1994) Wheat peptide challenge in celiac-disease. Lancet 343:758–761. doi:10.1016/S0140-6736(94)91837-6
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Vaccino P, Becker H, Brandolini A, Salamini F, Kilian B (2009) A catalogue of Triticum monococcum genes encoding toxic and immunogenic peptides for celiac disease patients. Mol Genet Genomics 281:289–300. doi:10.1007/s00438-008-0412-8
Vader W, Kooy Y, van Veelen P, De Ru A, Harris D, Benckhuijsen W, Pena S, Mearin L, Drijfhout JW, Koning F (2002) The gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology 122:1729–1737. doi:10.1053/gast.2002.33606
van de Wal Y, Kooy YM, van Veelen PA, Pena SA, Mearin LM, Molberg O, Lundin KE, Sollid LM, Mutis T, Benckhuijsen WE, Drijfhout JW, Koning F (1998) Small intestinal T cells of celiac disease patients recognize a natural pepsin fragment of gliadin. Proc Natl Acad Sci USA 95:10050–10054. doi:10.1073/pnas.95.17.10050
van Herpen TWJM, Goryunova SV, van der Schoot J, Mitreva M, Salentijn E, Vorst O, Schenk MF, van Veelen PA, Koning F, van Soest LJM, Vosman B, Bosch D, Hamer RJ, Gilissen LMWJ, Smulders MJM (2006) Alpha-gliadin genes from the A, B and D genomes of wheat contain different sets of celiac disease epitopes. BMC Genomics 7:1. doi:10.1186/1471-2164-7-1
Wang RRC, von Bothmer R, Dvorak J, Fedak G, Linde-Laursen I, Muramatsu M (1996) Genome symbols in the Triticeae (Poaceae). In: Wang RRC, Jensen KB, Jaussi C (eds) Proceedings of the 2nd international Triticeae Symposium. Logan, Utah, pp 29–34
Xie Z, Wang C, Wang K, Wang S, Li X, Zhang Z, Ma W, Yan Y (2010) Molecular characterization of the celiac disease epitope domains in α-gliadin genes in Aegilops tauschii and hexaploid wheats (Triticum aestivum L.). Theor Appl Genet 121:1239–1251. doi:10.1007/s00122-010-1384-8
Acknowledgments
The authors give special thanks to Prof. Yong-Hong Zhou and Deng-Cai Liu for providing the plant materials. The accessions with PI numbers were kindly provided by USDA-ARS (http://www.ars-grin.gov). The accessions with AS numbers were deposited at Triticeae Research Institute, Sichuan Agricultural University, China. This work was supported by the National Natural Science Foundation of China (31230053).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qi, PF., Chen, Q., Ouellet, T. et al. The molecular diversity of α-gliadin genes in the tribe Triticeae . Genetica 141, 303–310 (2013). https://doi.org/10.1007/s10709-013-9729-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-013-9729-2