Abstract
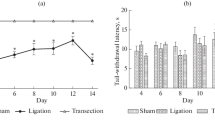
Pain and pain modulation has been viewed as being mediated entirely by neurons. However, new research implicates spinal cord glia as key players in the creation and maintenance of pathological pain. Sciatic nerve lesions are one of the most commonly studied pain-related injuries. In our study we aimed to characterize changes in microglial activation in the rat spinal cord after axotomy and chronic constriction injury of the sciatic nerve and to evaluate this activation in regard to pain behavior in injured and control groups of rats. Microglial activation was observed at ipsilateral side of lumbar spinal cord in all experimental groups. There were slight differences in the level and extent of microglial activation between nerve injury models used, however, differences were clear between nerve-injured and sham animals in accordance with different level of pain behavior in these groups. It is known that activated microglia release various chemical mediators that can excite pain-responsive neurons. Robust microglial activation observed in present study could therefore contribute to pathological pain states observed following nerve injury.




Similar content being viewed by others
References
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A (1998) Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391:281–285
Blinzinger K, Kreutzberg G (1968) Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie (Vienna, Austria: 1948) 85:145–157
Bruce-Keller AJ (1999) Microglial-neuronal interactions in synaptic damage and recovery. J Neurosci Res 58:191–201
Cizkova D, Lukacova N, Marsala M, Marsala J (2002) Neuropathic pain is associated with alterations of nitric oxide synthase immunoreactivity and catalytic activity in dorsal root ganglia and spinal dorsal horn. Brain Res Bull 58:161–171
Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF (1997) Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol 79:163–175
Colburn RW, Rickman AJ, DeLeo JA (1999) The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol 157:289–304
Dixon WJ (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20:441–162
Garrison CJ, Dougherty PM, Carlton SM (1994) GFAP expression in lumbar spinal cord of naive and neuropathic rats treated with MK-801. Exp Neurol 129:237–243
Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40:140–155
Hashizume H, DeLeo JA, Colburn RW, Weinstein JN (2000) Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine 25:1206–1217
Hatori K, Nagai A, Heisel R, Ryu JK, Kim SU (2002) Fractalkine and fractalkine receptors in human neurons and glial cells. J Neurosci Res 69:418–426
Haydon PG (2001) GLIA: listening and talking to the synapse. Nat Rev Neurosci 2:185–193
Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y (2000) Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem 75:965–972
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63
Imai Y, Kohsaka S (2002) Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia 40:164–174
Inoue A, Ikoma K, Morioka N, Kumagai K, Hashimoto T, Hide I, Nakata Y (1999) Interleukin-1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. J Neurochem 73:2206–2213
Jergova S, Cizkova D (2005) Long-term changes of c-Fos expression in the rat spinal cord following chronic constriction injury. Eur J Pain 9:345–354
Kommers T, Vinade L, Pereira C, Goncalves CA, Wofchuk S, Rodnight R (1998) Regulation of the phosphorylation of glial fibrillary acidic protein (GFAP) by glutamate and calcium ions in slices of immature rat spinal cord: comparison with immature hippocampus. Neurosci Lett 248:141–143
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318
Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR (2005) Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 115:71–83
Marriott DR, Wilkin GP, Wood JN (1991) Substance P-induced release of prostaglandins from astrocytes: regional specialisation and correlation with phosphoinositol metabolism. J Neurochem 56:259–265
Marriott I (2004) The role of tachykinins in central nervous system inflammatory responses. Front Biosci 9:2153–2165
Molina-Holgado F, Lledo A, Guaza C (1995) Evidence for cyclooxygenase activation by nitric oxide in astrocytes. Glia 15:167–172
Munglani R, Hudspith MJ, Fleming B, Harrisson S, Smith G, Bountra C, Elliot PJ, Birch PJ, Hunt SP (1999) Effect of pre-emptive NMDA antagonist treatment on long-term Fos expression and hyperalgesia in a model of chronic neuropathic pain. Brain Res 822:210–219
Nakajima K, Kohsaka S (2001) Microglia: activation and their significance in the central nervous system. J Biochem (Tokyo) 130:169–175
Narita M, Yoshida T, Nakajima M, Miyatake M, Takagi T, Yajima Y, Suzuki T (2006) Direct evidence for spinal cord microglia in the development of a neuropathic pain-like state in mice. J Neurochem 97:1337–1348
Palma C, Minghetti L, Astolfi M, Ambrosini E, Silberstein FC, Manzini S, Levi G, Aloisi F (1997) Functional characterization of substance P receptors on cultured human spinal cord astrocytes: synergism of substance P with cytokines in inducing interleukin-6 and prostaglandin E2 production. Glia 21:183–193
Raghavendra V, Tanga F, DeLeo JA (2003) Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 306:624–630
Rasley A, Bost KL, Olson JK, Miller SD, Marriott I (2002) Expression of functional NK-1 receptors in murine microglia. Glia 37:258–267
Ro LS, Li HY, Huang KF, Chen ST (2004) Territorial and extra-territorial distribution of Fos protein in the lumbar spinal dorsal horn neurons in rats with chronic constriction nerve injuries. Brain Res 1004:177–187
Stoll G, Jander S (1999) The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol 58:233–247
Streit WJ (2002) Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 40:133–139
Stuesse SL, Cruce WLR, Lovell JA, McBurney DL, Crisp T (2000) Microglial proliferation in the spinal cord of aged rats with a sciatic nerve injury. Neuroscience Letters 287:121–124
Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J (2001) Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 21:2580–2588
Tikka TM, Koistinaho JE (2001) Minocycline provides neuroprotection against N-methyl-d-aspartate neurotoxicity by inhibiting microglia. J Immunol 166:7527–7533
Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC (2004) Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci 20:1150–1160
Wall PD, Devor M (1983) Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain 17:321–339
Watkins LR, Milligan ED, Maier SF (2001a) Glial activation: a driving force for pathological pain. Trends Neurosci 24:450–455
Watkins LR, Milligan ED, Maier SF (2001b) Spinal cord glia: new players in pain. Pain 93:201–205
Willis WD, Coggeshall RE (1991) Sensory mechanisms of the spinal cord. Plenum Press, New York
Winkelstein BA, DeLeo JA (2002) Nerve root injury severity differentially modulates spinal glial activation in a rat lumbar radiculopathy model: considerations for persistent pain. Brain Res 956:294–301
Woolf CJ, Salter MW (2000) Neuronal plasticity: increasing the gain in pain. Science 288:1765–1769
Yamazaki Y, Maeda T, Someya G, Wakisaka S (2001) Temporal and spatial distribution of Fos protein in the lumbar spinal dorsal horn neurons in the rat with chronic constriction injury to the sciatic nerve. Brain Res 914:106–114
Zhang J, Koninck Y (2006) Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem 97:772–783
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110
Acknowledgements
The authors thank to M. Spontakova for excellent technical assistance. This work was supported by Science and Technology Assistance Agency under the contract No. APVT-51-011604 and by grant of Slovak Academy of Sciences VEGA No. 2/5136/25.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jergová, S., Čížková, D. Microglial activation in different models of peripheral nerve injury of the rat. J Mol Hist 38, 245–251 (2007). https://doi.org/10.1007/s10735-007-9094-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-007-9094-5