Abstract
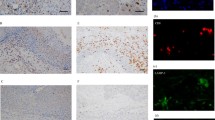
Signals from the T cell immunoglobulin and mucin-domain (TIM)-containing molecules have been demonstrated to be involved in regulating the progress of carcinoma. However, the expression and anatomical distribution of TIMs in Langerhans cell sarcoma (LCS), which is a rare malignancy derived from dendritic cells of the epidermis, has yet to be determined. In this study, the expression of TIM-1, TIM-3 and TIM-4 in LCS samples were detected by immunohistochemistry. Our results showed that these three molecules were found in LCS sections. At the cellular level, these molecules were found on the cell membrane and in the cytoplasm. Immunofluorescence double-staining demonstrated that these TIMs were co-expressed with Langerin, a potential biomarker for detecting LCS. In addition, TIM-1 was also expressed on CD68+ macrophages and CK-18+ epithelial cells, while TIM-3 and TIM-4 were expressed on all cell types investigated, including CD3+T cells, CD68+ macrophages, CD11c+ dendritic cells, CD16+ NK Cells, CD31+ endothelial cells and CK-18+ epithelial cells. Interestingly, TIMs were also co-expressed with some members of the B7 superfamily, including B7-H1, B7-H3 and B7-H4 on sarcoma cells. Our results clearly showed the characteristic expression and anatomical distribution of TIMs in LCS, and a clear understanding of their functional roles may further elucidate the pathogenesis of this carcinoma and potentially contribute to the development of novel immunotherapeutic strategies.




Similar content being viewed by others
References
Anderson AC (2012) Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol 24(2):213–216
Chiba S, Baghdadi M, Akiba H et al (2012) Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol 13(9):832–842
Freeman GJ, Casasnovas JM, Umetsu DT et al (2010) TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev 235(1):172–189
Hashiguchi M, Kobori H, Ritprajak P et al (2008) Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7–H3 and enhances T cell responses. Proc Natl Acad Sci USA 105(2008):10495–10500
He W, Fang Z, Wang F et al (2010) Galectin-9 significantly prolongs the survival of fully mismatched cardiac allografts in mice. Transplantation 88(6):782–790
Huang X, Bai X, Cao Y et al (2010) Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J Exp Med 207(3):505–520
Lau SK, Chu PG, Weiss LM (2008) Immunohistochemical expression of Langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol 32(4):615–619
Li H, Wang C, Guo G et al (2012a) The characteristic expression of B7-associated proteins in Langerhans cell sarcoma. Acta Histochem 114(7):733–743
Li H, Wu K, Tao K et al (2012b) Tim-3/galectin-9 signaling pathway mediates T cell dysfunction and predicts poor prognosis in patients with HBV-associated hepatocellular carcinoma. Hepatology
Miyanishi M, Tada K, Koike M et al (2007) Identification of Tim4 as a phosphatidylserine receptor. Nature 450(7168):435–439
Monney L, Sabatos CA, Gaglia JL et al (2002) Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415(6871):536–541
Nakayama M, Akiba H, Takeda K et al (2009) Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 113(16):3821–3830
Nakayama M, Takahashi K, Hori M et al (2010) Langerhans cell sarcoma of the cervical lymph node: a case report and literature review. Auris Nasus Larynx 37(6):750–753
Nozaki Y, Nikolic-Paterson DJ, Snelgrove SL et al (2012) Endogenous Tim-1 (Kim-1) promotes T-cell responses and cell-mediated injury in experimental crescentic glomerulonephritis. Kidney Int 81(9):844–855
Rennert PD (2011) Novel roles for TIM-1 in immunity and infection. Immunol Lett 141(1):28–35
Rodriguez-Manzanet R, DeKruyff R, Kuchroo VK et al (2009) The costimulatory role of TIM molecules. Immunol Rev 229(1):259–270
Rodriguez-Manzanet R, Sanjuan MA, Wu HY et al (2010) T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc Natl Acad Sci USA 107(19):8706–8711
Sánchez-Fueyo A, Tian J, Picarella D et al (2003) Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol 4(11):1093–1101
Saresella M, Rainone V, Al-Daghri NM et al (2012) The PD-1/PD-L1 pathway in human pathology. Curr Mol Med 12(3):259–267
Seki M, Oomizu S, Sakata KM et al (2008) Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol 127(1):78–88
Sica GL, Choi IH, Zhu G et al (2003) B7–H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 18(6):849–861
Sonar SS, Hsu YM, Conrad ML et al (2010) Antagonism of TIM-1 blocks the development of disease in a humanized mouse model of allergic asthma. J Clin Invest 120(8):2767–2781
Uchida K, Kobayashi S, Inukai T et al (2008) Langerhans cell sarcoma emanating from the upper arm skin: successful treatment by MAID regimen. J Orthop Sci 13:89–93
Wong K, Valdez PA, Tan C et al (2010) Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc Natl Acad Sci USA 107(19):8712–8717
Yang PC, Xing Z, Berin CM et al (2007) TIM-4 expressed by mucosal dendritic cells plays a critical role in food antigen-specific Th2 differentiation and intestinal allergy. Gastroenterology 133(5):1522–1533
Zhuang X, Zhang X, Xia X et al (2012) Ectopic expression of TIM-3 in lung cancers: a potential independent prognostic factor for patients with NSCLC. Am J Clin Pathol 137(6):978–985
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (NSFC No. 81171585, 30971099 and 61141012) and Natural Science Foundation of Chongqing (No CSTC2011BB5037).
Conflicts of interest
None of the authors have any conflicts of interest related to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jingwei Li and Dayan Cao equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10735_2012_9475_MOESM1_ESM.tif
Supplmental Figure 1 The morphology of TIM-1 positive cells in LCS sample sections detected by immunofluorescence double-staining. Immunofluorescence double-staining showed that TIM-1 was expressed on CD68+ macrophages and CK-18+ epithelial cells, while it was absent on CD3+ T cells, CD11c+DCs, CD16+ monocytes, and CD31+ endothelial cells. Arrow indicates positive cells. Nuclei were stained with DAPI. Scale bar = 20 μm. (TIFF 6903 kb)
10735_2012_9475_MOESM2_ESM.tif
Supplmental Figure 2 The morphology of TIM-3positive cells in LCS sample sections detected by immunofluorescence double-staining. Immunofluorescence double-staining showed that TIM-3 was expressed on CD3+ T cells, CD68+ macrophages, CD11c+ dendritic cells, CD16+ monocytes, CD31+ endothelial cells and CK-18+ epithelial cells. Arrow indicates positive cells. Nuclei were stained with DAPI. Scale bar = 20 μm. (TIFF 7448 kb)
10735_2012_9475_MOESM3_ESM.tif
Supplmental Figure 3 The morphology of TIM-4positive cells in LCS sample sections was detected by immunofluorescence double-staining. Immunofluorescence double-staining showed that TIM-4 was expressed on CD3+ T cells, CD68+ macrophages, CD11c+ dendritic cells, CD16+ monocytes, CD31+ endothelial cells and CK-18+ epithelial cells. Arrow indicates positive cells. Nuclei were stained with DAPI. Scale bar = 20 μm. (TIFF 5579 kb)
Rights and permissions
About this article
Cite this article
Li, J., Cao, D., Guo, G. et al. Expression and anatomical distribution of TIM-containing molecules in Langerhans cell sarcoma. J Mol Hist 44, 213–220 (2013). https://doi.org/10.1007/s10735-012-9475-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-012-9475-2