Abstract
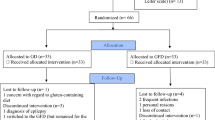
To obtain information on the safety and efficacy of the gluten-free/casein-free (GFCF) diet, we placed 14 children with autism, age 3–5 years, on the diet for 4–6 weeks and then conducted a double-blind, placebo-controlled challenge study for 12 weeks while continuing the diet, with a 12-week follow-up. Dietary challenges were delivered via weekly snacks that contained gluten, casein, gluten and casein, or placebo. With nutritional counseling, the diet was safe and well-tolerated. However, dietary challenges did not have statistically significant effects on measures of physiologic functioning, behavior problems, or autism symptoms. Although these findings must be interpreted with caution because of the small sample size, the study does not provide evidence to support general use of the GFCF diet.



Similar content being viewed by others
References
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: Author.
Bock, S. A., Sampson, H. A., Atkins, F. M., Zeiger, R. S., Lehrer, S., Sachs, M., & Metcalfe, D. D. (1988). Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. Journal of Allergy and Clinical Immunology, 82(6), 986–997.
Bootzin, R., Buysse, D., Edinger, J., Espie, C., Lichstein, K., & Morin, C. (2006). Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004). Sleep, 29, 1398–1414.
Buie, T., Campbell, D. B., Fuchs, G. J, I. I. I., Furuta, G. T., Levy, J., Van de Water, J., & Winter, H. (2010). Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A consensus report. Pediatrics, 125(Supplement 1), S1–S18.
Catassi, C., Elli, L., Bonaz, B., Bouma, G., Carroccio, A., Castillejo, G., & Fasano, A. (2015). Diagnosis of non-celiac gluten sensitivity (NCGS): The Salerno experts’ criteria. Nutrients, 7(6), 4966.
Centers for Disease Control and Prevention (2014). National Health and Nutrition Examination Survey, 2013–2014. Retrieved from http://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
Conners, C. K. (1990). Conners’ Abbreviated Symptom Questionnaire: Parent version, teacher version manual. Toronto: Multi-Health Systems.
Diggle, P. J., Heagerty, P., Liang, K. Y., & Zeger, S. L. (2002). Analysis of longitudinal data (2nd ed.). New York: Oxford University Press.
Elder, J. H., Shankar, M., Shuster, J., Theriaque, D., Burns, S., & Sherrill, L. (2006). The gluten-free, casein-free diet in autism: Results of a preliminary double blind clinical trial. Journal of Autism and Developmental Disorders, 36, 413–420.
ESHA Research (2014). Food Processor Nutrition Analysis and Fitness Software: Full Manual. Retrieved from http://www.esha.com/sites/default/files/documents/full_manual.pdf.
Filipek, P. A., Accardo, P. J., Baranek, G. T., Cook, E. H, Jr, Dawson, G., Gordon, B., & Volkmar, F. R. (1999). The screening and diagnosis of autistic spectrum disorders. Journal of Autism and Developmental Disorders, 29, 439–484.
Freeman, B. J., Ritvo, E. R., Yokota, A., & Ritvo, A. (1986). A scale for rating symptoms of patients with the syndrome of autism in real-life settings. Journal of the American Academy of Child and Adolescent Psychiatry, 25, 131–136.
GFCFDiet (2001). Parent survey GFCF diet. Accessed November 6, 2014, at http://www.gfcfdiet.com/dietsurveysept2.htm.
Halmos, E. P., Christophersen, C. T., Bird, A. R., Shepherd, S. J., Gibson, P. R., & Muir, J. G. (2015). Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut, 64(1), 93–100. doi:10.1136/gutjnl-2014-307264.
Hollingshead, A. B. (1975). Four-factor index of social status. Retrieved from http://elsinore.cis.yale.edu/sociology/yjs/yjs_fall_2011.pdf#page=21.
Horvath, K., Papadimitriou, J. C., Rabsztyzn, A., Drachenberg, C., & Tildon, J. T. (1999). Gastrointestinal abnormalities in children with autistic disorder. Journal of Pediatrics, 135, 559–563.
Horvath, K., & Perman, J. A. (2002). Autistic disorder and gastrointestinal disease. Current Opinion in Pediatrics, 14, 583–587.
Hsu, J. C. (1992). The factor analytic approach to simultaneous inference in the general linear model. Journal of Computational and Graphical Statistics, 1(2), 151–168. doi:10.1080/10618600.1992.10477011.
Hyman, S. L., Stewart, P. A., Schmidt, B., Lemcke, N., Foley, J. T., Peck, R., & Handen, B. (2012). Nutrient intake from food in children with autism. Pediatrics, 130(Supplement 2), S145–S153. doi:10.1542/peds.2012-0900L.
Interactive Autism Network (IAN, 2008). IAN Research Findings: Special Diets. Retrieved from http://www.iancommunity.org/cs/ian_treatment_reports/special_diets.
Johnson, C. R., Handen, B. L., Zimmer, M., Sacco, K., & Turner, K. (2011). Effects of gluten free/casein free diet in young children with autism: A pilot study. Journal of Developmental and Physical Disabilities, 23(3), 213–225. doi:10.1007/s10882-010-9217-x.
Kemperman, R., Muskiet, F., Boutier, A., Kema, I., & Muskiet, F. (2008). Normal intestinal permeability at elevated platelet serotonin levels in a subgroup of children with pervasive developmental disorders in Curacao (the Netherlands Antilles). Journal of Autism and Developmental Disorders, 38, 401–406. doi:10.1007/s10803-007-0399-8.
Knivsberg, A. M., Reichelt, K. L., Høien, T., & Nødland, M. (2002). A randomised, controlled study of dietary intervention in autistic syndromes. Nutritional Neuroscience, 5(4), 251–261.
Knivsberg, A. M., Reichelt, K. L., Nødland, M., & Høien, T. (1995). Autistic syndromes and diet: A follow-up study. Scandinavian Journal of Educational Research, 39(3), 222–236.
Lewis, L. (2011). Special diets for special kids, Volumes 1 and 2 combined: Over 200 revised and new gluten-free casein-free recipes, plus research on the positive effects for children with autism, ADHD, allergies, celiac disease, and more!. Arlington, TX: Future Horizons.
Lewis, S. J., & Heaton, K. W. (1997). Stool form scale as a useful guide to intestinal transit time. Scandinavian Journal of Gastroenterology, 32(9), 920–924. doi:10.3109/00365529709011203.
Lord, C., Rutter, M., DiLavore, P. C., & Risi, S. (2003). Autism diagnostic observation schedule. Los Angeles, CA: Western Psychological Services.
Matthews, J. (2008). Nourishing hope for children with autism: Nutrition and diet guide for healing our children. San Francisco: Healthful Living Media.
McElhanon, B. O., McCracken, C., Karpen, S., & Sharp, W. G. (2014). Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics, 133(5), 872–883.
Metcalfe, D. D., & Sampson, H. A. (1990). Workshop on experimental methodology for clinical studies of adverse reactions to foods and food additives. Journal of Allergy and Clinical Immunology, 86(3), 421–442.
Millward, C., Ferriter, M., Calver, S. J., & Connell-Jones, G. G. (2008). Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database of Systematic Reviews,. doi:10.1002/14651858.CD003498.pub3.
Mullen, E. M. (1995). Mullen Scales of early learning. Circle Pines, MN: American Guidance Service.
Mulloy, A., Lang, R., O’Reilly, M., Sigafoos, J., Lancioni, G., & Rispoli, M. (2010). Gluten-free and casein-free diets in the treatment of autism spectrum disorders: A systematic review. Research in Autism Spectrum Disorders, 4(3), 328–339.
Murray, J. A., Watson, T., Clearman, B., & Mitros, F. (2004). Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. American Journal of Clinical Nutrition, 79(4), 669–673.
Nowak-Wegrzyn, A., Assa’ad, A. H., Bahna, S. L., Bock, S. A., Sicherer, S. H., & Teuber, S. S. (2009). Work Group report: Oral food challenge testing. Journal of Allergy and Clinical Immunology, 123(6 Supplement), S365–S383. doi:10.1016/j.jaci.2009.03.042.
Perrin, J. M., Coury, D. L., Hyman, S. L., Cole, L., Reynolds, A. M., & Clemons, T. (2012). Complementary and alternative medicine use in a large pediatric autism sample. Pediatrics, 130(Supplement 2), S77–S82. doi:10.1542/peds.2012-0900E.
Reichelt, K. L., Knivsberg, A., Lind, G., & Nødland, M. (1991). Probable etiology and possible treatment of childhood autism. Brain Dysfunction, 4, 308–319.
Reichelt, K. L., Knivsberg, A., Nødland, M., & Lind, G. (1994). Nature and consequences of hyperpeptiduria and bovine casomorphins found in autistic syndromes. Developmental Brain Dysfunction, 7, 71–85.
Reichelt, K. L., & Landmark, J. (1995). Specific IgA antibody increases in schizophrenia. Journal of Biological Psychiatry, 37, 410–413.
Robertson, M., Sigalet, D., Holst, J., Meddings, J., Wood, J., & Sharkey, K. (2008). Intestinal permeability and glucagon-like peptide-2 in children with autism: A controlled pilot study. Journal of Autism and Developmental Disorders, 38, 1066–1071. doi:10.1007/s10803-007-0482-1
Robins, J. M., Rotnitzky, A., & Zhao, L. P. (1995). Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. Journal of the American Statistical Association, 90, 106–121.
Rutter, M., Le Couteur, A., & Lord, C. (2003). Autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services.
Seroussi, K. (2002). Unraveling the mystery of autism and pervasive developmental disorder: A mother’s story of research & recovery. New York, NY: Crown.
Sevin, J. A., Matson, J. L., Coe, D. A., Fee, V. E., & Sevin, B. M. (1991). A comparison and evaluation of three commonly used autism scales. Journal of Autism and Developmental Disorder, 21, 417–432.
Sicherer, S. H. (1999). Food allergy: When and how to perform oral food challenges. Pediatric Allergy and Immunology, 10(4), 226–234.
Smith, T., Klorman, R., & Mruzek, D. W. (2015). Predicting outcome of community-based early intensive behavioral intervention for children with autism. Journal of Abnormal Child Psychology,. doi:10.1007/s10802-015-0002-2.
Sparrow, S. S., Balla, D. A., & Cicchetti, D. V. (1984). Vineland Adaptive Behavior Scales (Survey ed.). Circle Pines, MN: American Guidance Service.
Stewart, P. A., Hyman, S. L., Schmidt, B. L., Macklin, E. A., Reynolds, A., Johnson, C. R., et al. (2015). Dietary supplementation in children with autism spectrum disorders: Common, insufficient and excessive. Journal of the Academy of Nutrition and Dietetics, 115, 1237–1248. doi:10.1016/j.jand.2015.03.026.
Talk About Curing Autism (2010). Diet infringement or infraction help. https://www.tacanow.org/family-resources/diet-infringement-or-infraction-help/. Accessed 21 Aug 2015.
Whiteley, P., Haracopos, D., Knivsberg, A. M., Reichelt, K. L., Parlar, S., Jacobsen, J., & Shattock, P. (2010). The ScanBrit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutritional Neuroscience, 13(2), 87–100. doi:10.1179/147683010x12611460763922.
Whiteley, P., Rodgers, J., Savery, D., & Shattock, P. (1999). A gluten-free diet as an intervention for autism and associated spectrum disorders: Preliminary findings. Autism, 3(1), 45–65. doi:10.1177/1362361399003001005.
WHO Multicentre Growth Reference Study Group. (2006). WHO child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva, World Health Organization, 2006. Retrieved from http://www.who.int/childgrowth/standards/Technical_report.pdf?ua=1.
Williams, K. M., & Marshall, T. (1992). Urinary protein patterns in autism as revealed by high resolution two-dimensional electrophoresis. Biochemical Society Transactions, 20, 189S.
Acknowledgments
The project described in this publication was funded by U54 MH066397 (NIH/NIMH Genotype and Phenotype of Autism, Principal Investigator: Patricia Rodier). It was also supported by the University of Rochester CTSA award UL1 RR024160 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the children and families who participated. We also thank Eileen Blakely, R.D., for assisting with nutritional monitoring; Emily Healy for assisting with data collection; Patricia Rodier, Ph.D. (deceased), for her leadership of the STAART Center; Caroline Magyar, Ph.D., and her staff for conducting intake assessments of participants; Christopher Stodgell, Ph.D., for directing data management of the project; and Loisa Bennetto, Ph.D., for her advice. We are grateful to the following agencies who referred participants to the study, participated in study implementation, and advised us: Stepping Stones Learning Center (Mariellen Cupini, Director; Dr. John McEachin, EIBI Consultant) and the Center for Autism and Related Disorders (Fairport, NY branch, Director: Denise Rhine; Dr. Doreen Granpeesheh, Executive Director).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hyman, S.L., Stewart, P.A., Foley, J. et al. The Gluten-Free/Casein-Free Diet: A Double-Blind Challenge Trial in Children with Autism. J Autism Dev Disord 46, 205–220 (2016). https://doi.org/10.1007/s10803-015-2564-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-015-2564-9