Abstract
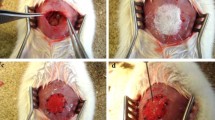
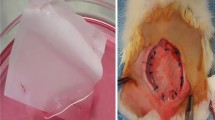
The aim of this study was to engineer skeletal muscle tissue for repair abdominal wall defects. Myoblast were seeded onto the scaffolds and cultivated in vitro for 5 days. Full thickness abdominal wall defects (3 × 4 cm) were created in 18 male New Zealand white rabbits and randomly divided into two equal groups. The defects of the first group were repaired with myoblast-seeded-bovine tunica vaginalis whereas the second group repaired with non-seeded-bovine tunica vaginalis and function as a control. Three animals were sacrificed at 7th, 14th, and 30th days of post-implantation from each group and the explanted specimens were subjected to macroscopic and microscopic analysis. In every case, seeded scaffolds have better deposition of newly formed collagen with neo-vascularisation than control group. Interestingly, multinucleated myotubes and myofibers were only detected in cell-seeded group. This study demonstrated that myoblast-seeded-bovine tunica vaginalis can be used as an effective scaffold to repair severe and large abdominal wall defects with regeneration of skeletal muscle tissue.






Similar content being viewed by others
References
Mooney DJ, Mikos AG. Growing new organs. Sci Am. 1999;280:60–5.
Law PK, Goodwin TG, Fang Q, Deering MB, Duggirala V, Larkin C, et al. Cell transplantation as an experimental treatment for Duchenne muscular dystrophy. Cell Transplant. 1993;2:485–505.
Guettier-Sigrist S, Coupin G, Braun S, Warter JM, Poindron P. Muscle could be the therapeutic target in SMA treatment. J Neurosci Res. 1998;53:663–9.
Conconi MT, Coppi PD, Bellini S, Zara G, Sabatti M, Marzaro M, et al. Homologous muscle acellular matrix seeded with autologous myoblasts as a tissue-engineering approach to abdominal wall-defect repair. Biomaterials. 2005;16:2567–74.
Lai JY, Chang PY, Lin JN. Body wall repair using small intestinal submucosa seeded with cells. J Pediatr Surg. 2003;38:1752–5.
Frandson RD, Wilke LW, Fails DA. Anatomy and physiology of farm animals. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2003.
Hafeez YM, Zuki ABZ, Loqman MY, Noordin MM, Norimah Y. Comparative evaluations of the processed bovine tunica vaginalis implant in a rat model. Anat Sci Int. 2005;80:181–8.
Springer ML, Rando T, Blau HM. Gene delivery to muscle. In: Boyle AL, editor. Current protocols in human genetics. Unit 13.4. New York: Wiley; 1997. p. 13.4.1–19.
Pavlath GK. Isolation, purification and growth of human skeletal muscle cells. In: Jones GE, editor. Methods in molecular medicine: human cell culture protocols. Totowa: Humana Press; 1996. p. 307–17.
Gangwar AK, Sharma AK, Kumar N, Kumar N, Maiti SK, Gupta OP, et al. Acellular dermal graft for repair of abdominal wall defects in rabbits. J S Afr Vet Assoc. 2006;77:79–85.
Singh J, Kumar N, Sharma AK, Maiti SK, Goswami TK, Sharma AK. Acellular biomaterials of porcine origin for the reconstruction of abdominal wall defects in rabbits. Trends Biomater Artif Organs. 2008;22:34–44.
Jenkins SD, Klamer TW, Parteka JJ, Condon RE. A comparison of prosthetic materials used to repair abdominal wall defects. Surgery. 1983;94:392–8.
Tung WS, Zainol J, Pillay AG, Yusof N, Yusof LM. Processed bovine tunica vaginalis as a biomaterial for the repair of large abdominal wall defects in surgical treatment. Science. 2002;2:7–11.
Mattioli-Belmonte M, Lucarini G, Zizzi A, Alhuwalia A, Vozzi G. A comparative study of porous and engineered biomaterials. Biomed Pharmacother. 2008;62:487–502.
Prokop A, Rosenberg MZ. Bioreactor for mammalian cell culture. Adv Biochem Eng Biotechnol. 1989;39:29–71.
Kim MS, Ahn HH, Shin YN, Cho MH, Khang G, Lee HB. An in vivo study of the host tissue response to subcutaneous implantation of PLGA- and/or porcine small intestinal submucosa-based scaffolds. Biomaterials. 2007;28:5137–43.
Zuki ABZ, Hafeez YM, Loqman MY, Noordin MM, Norimah Y. Effect of preservation methods on the performance of bovine pericardium grafts in a rat model. Anat Histol Embryol. 2007;36:349–56.
Decurtins M, Buchman P. Bovine pericardium-A new graft material for hernial repair. Res Exp Med. 1982;180:11–4.
Badylak S, Kokini K, Tullius B, Simmons-Byrd A, Morff R. Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res. 2002;103:190–202.
Chung S, Hazen A, Levine JP, Baux G, Olivier WA, Yee HT, et al. Vascularized acellular dermal matrix island flaps for the repair of abdominal muscle defects. Plast Reconstr Surg. 2003;111:225–32.
Wei CY, Chuang DC, Chen HC, Lin CH, Wong SS, Wei FC. The versatility of free rectus femoris muscle flap an alternative flap. Microsurgery. 1995;16:698–703.
Fauza DO, Marler JJ, Koka R, Forse RA, Mayer JE, Vacanti JP. Fetal tissue engineering: diaphragmatic replacement. J Pediatr Surg. 2001;36:146–51.
Silver IA. Basic physiology of wound healing in horse. Equine Vet J. 1982;14:7–15.
Gamba PG, Conconi MT, Piccolo RL, Zara G, Spinazzi R, Parnigotto PP. Experimental abdominal wall defect repaired with acellular matrix. Pediatr Surg Int. 2002;18:327–31.
Van Wachem PB, Brouwer LA, van Luyn MJA. Absence of muscle regeneration after implantation of a collagen matrix seeded with myoblasts. Biomaterials. 1999;20:419–26.
Kamelger FS, Marksteiner R, Margreiter E, Klima G, Wechselberger G, Hering S, et al. A comparative study of three different biomaterials in the engineering of skeletal muscle using a rat animal model. Biomaterials. 2004;25:1649–55.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ayele, T., Zuki, A.B.Z., Noorjahan, B.M.A. et al. Tissue engineering approach to repair abdominal wall defects using cell-seeded bovine tunica vaginalis in a rabbit model. J Mater Sci: Mater Med 21, 1721–1730 (2010). https://doi.org/10.1007/s10856-010-4007-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-010-4007-7