Abstract
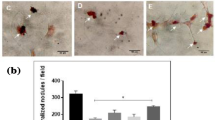
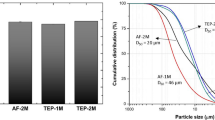
To validate the feasibility of two types of bioactive glass that contains spherical and radical spherical nano-sized particles in promoting bone repair, we hypothesize that radical spherical nano-sized particles have higher bone repair effectiveness than spherical one due to the physicochemical properties. We rigorously compared the physicochemical properties and bioactivities of these two types of bioactive glass. Specifically, we measured the size, surface morphology, concentration of ionic-dissolution products, bioactivity, and biological effects of two groups of bioactive glass on rat bone marrow mesenchymal stem cells (rBMSCs) and evaluate their effect on proliferation and osteogenic differentiation of rBMSCs in vitro. We observed that spherical nano-bioactive glass (SNBG) was spherical with smooth boundary, while the radial spherical nano-bioactive glass (RSNBG) had radial pore on the surface of particle boundary. When the two materials were immersed in simulated body fluid for 24 h, RSNBG produced more and denser hydroxyapatite carbonate than SNBG. The concentration of Ca and Si ions in RSNBG 24 h extract is higher than that of SNBG, while the concentration of P ions is lower. Proliferation, alkaline phosphatase (ALP) activity, intracellular Ca ion concentrations defined as the number of mineralized nodules produced, and the expression of osteogenic genes were significantly higher in rBMSCs co-cultured with 50 µg/mL RSNBG than SNBG. Overall, these results validated our hypothesis that RSNBG can provide better benefit than SNBG for inducing proliferation and osteogenic differentiation in rBMSCs, in turn suggested the feasibility of this RSNBG in further studies and utilization toward the ends of improved bone repair effectiveness.









Similar content being viewed by others
References
Drago L, Toscano M, Bottagisio M. Recent evidence on bioactive glass antimicrobial and antibiofilm activity: a mini-review. Materials. 2018;11:326. https://doi.org/10.3390/ma11020326.
Buser Z, Brodke DS, Youssef JA, Meisel HJ, Myhre SL, Hashimoto R, et al. Synthetic bone graft versus autograft or allograft for spinal fusion: a systematic review. J Neurosurg Spine. 2016;25:509–16. https://doi.org/10.3171/2016.1.SPINE151005.
Ho-Shui-Ling A, Bolander J, Rustom LE, Johnson AW, Luyten FP, Picart C. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143–62. https://doi.org/10.1016/j.biomaterials.2018.07.017.
Iaquinta MR, Mazzoni E, Manfrini M, D’Agostino A, Trevisiol L, Nocini R, et al. Innovative biomaterials for bone regrowth. Int J Mol Sci. 2019;20:618. https://doi.org/10.3390/ijms20030618.
Bellucci D, Salvatori R, Anesi A, Chiarini L, Cannillo V. SBF assays, direct and indirect cell culture tests to evaluate the biological performance of bioglasses and bioglass-based composites: three paradigmatic cases. Mater Sci Eng C Mater Biol Appl. 2019;96:757–64. https://doi.org/10.1016/j.msec.2018.12.006.
Thomas A, Bera J. Preparation and characterization of gelatin-bioactive glass ceramic scaffolds for bone tissue engineering. J Biomater Sci Polym Ed. 2019;30:561–79. https://doi.org/10.1080/09205063.2019.1587697.
Granel H, Bossard C, Nucke L, Wauquier F, Rochefort GY, Guicheux J, et al. Optimized bioactive glass: the quest for the bony graft. Adv Healthc Mater. 2019;8:e1801542. https://doi.org/10.1002/adhm.201801542.
Hench LL, Splinter RJ, Allen WC, Greenlee TK. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res A. 1971;5:117–41. https://doi.org/10.1002/jbm.820050611.
Su TR, Chu YH, Yang HW, Huang YF, Ding SJ. Component effects of bioactive glass on corrosion resistance and in vitro biological properties of apatite-matrix coatings. Biomed Mater Eng. 2019;30:207–18. https://doi.org/10.3233/Bme-191045.
Ojansivu M, Wang X, Hyvari L, Kellomaki M, Hupa L, Vanhatupa S, et al. Bioactive glass induced osteogenic differentiation of human adipose stem cells is dependent on cell attachment mechanism and mitogen-activated protein kinases. Eur Cell Mater. 2018;35:54–72. https://doi.org/10.22203/eCM.v035a05.
Baino F, Novajra G, Miguez-Pacheco V, Boccaccini AR, Vitale-Brovarone C. Bioactive glasses: special applications outside the skeletal system. J Non Cryst Solids. 2016;432:15–30. https://doi.org/10.1016/j.jnoncrysol.2015.02.015.
Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J Biomed Mater Res. 2001;55:151–7. https://doi.org/10.1002/1097-4636(200105)55:2<151::Aid-Jbm1001>3.3.Co;2-4.
Hoppe A, Guldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–74. https://doi.org/10.1016/j.biomaterials.2011.01.004.
Zhang D, Lepparanta O, Munukka E, Ylanen H, Viljanen MK, Eerola E, et al. Antibacterial effects and dissolution behavior of six bioactive glasses. J Biomed Mater Res A. 2010;93a:475–83. https://doi.org/10.1002/jbm.a.32564.
Ajita J, Saravanan S, Selvamurugan N. Effect of size of bioactive glass nanoparticles on mesenchymal stem cell proliferation for dental and orthopedic applications. J Biomed Mater Res A. 2015;53:142–9. https://doi.org/10.1016/j.msec.2015.04.041.
Srinivasan S, Jayasree R, Chennazhi KP, Nair SV, Jayakumar R. Biocompatible alginate/nano bioactive glass ceramic composite scaffolds for periodontal tissue regeneration. Carbohydr Polym. 2012;87:274–83. https://doi.org/10.1016/j.carbpol.2011.07.058.
Mačković M, Hoppe A, Detsch R, Mohn D, Stark WJ, Spiecker E, et al. Bioactive glass (type 45S5) nanoparticles: in vitro reactivity on nanoscale and biocompatibility. J Nanopart Res. 2012;14:966. https://doi.org/10.1007/s11051-012-0966-6.
Vichery C, Nedelec JM. Bioactive glass nanoparticles: from synthesis to materials design for biomedical applications. Materials. 2016;9:288. https://doi.org/10.3390/ma9040288.
Greenspan’ DC, Zhong JP, Chen XF, LaTorre GP. The evaluation of degradability of melt and sol-gel derived Bioglass® in vitro. Bioceramics. 1997;10:391–4. https://doi.org/10.1016/B978-008042692-1/50093-5.
Suh WH, Suslick KS, Stucky GD, Suh YH. Nanotechnology, nanotoxicology, and neuroscience. Prog Neurobiol. 2009;87:133–70. https://doi.org/10.1016/j.pneurobio.2008.09.009.
Simchi A, Tamjid E, Pishbin F, Boccaccini AR. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomedicine. 2011;7:22–39. https://doi.org/10.1016/j.nano.2010.10.005.
Bush JR, Liang HX, Dickinson M, Botchwey EA. Xylan hemicellulose improves chitosan hydrogel for bone tissue regeneration. Polym Adv Technol. 2016;27:1050–5. https://doi.org/10.1002/pat.3767.
Wang SN, Gao XJ, Gong WY, Zhang ZC, Chen XF, Dong YM. Odontogenic differentiation and dentin formation of dental pulp cells under nanobioactive glass induction. Acta Biomater. 2014;10:2792–803. https://doi.org/10.1016/j.actbio.2014.02.013.
Wang YD, Liao TS, Shi M, Liu C, Chen XF. Facile synthesis and in vitro bioactivity of radial mesoporous bioactive glasses. Mater Lett. 2017;206:205–9. https://doi.org/10.1016/j.matlet.2017.07.021.
Li YL, Liang QM, Lin C, Li X, Chen XF, Hu Q. Facile synthesis and characterization of novel rapid-setting spherical sub-micron bioactive glasses cements and their biocompatibility in vitro. Mater Sci Eng C Mater Biol Appl. 2017;75:646–52. https://doi.org/10.1016/j.msec.2017.02.095.
Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295:1014–7. https://doi.org/10.1126/science.1067404.
Salinas AJ, Martin AI, Vallet-Regi M. Bioactivity of three CaO-P2O5-SiO2 sol-gel glasses. J. Biomed Mater Res. 2002;61:524–32. https://doi.org/10.1002/jbm.10229.
Baino F, Hamzehlou S, Kargozar S. Bioactive glasses: where are we and where are we going. J Funct Biomater. 2018;9:25 https://doi.org/10.3390/jfb9010025.
Jones JR. New trends in bioactive scaffolds: the importance of nanostructure. J Eur Ceram Soc. 2009;29:1275–81. https://doi.org/10.1016/j.jeurceramsoc.2008.08.003.
Misra SK, Mohn D, Brunner TJ, Stark WJ, Philip SE, Roy I, et al. Comparison of nanoscale and microscale bioactive glass on the properties of P(3HB)/Bioglass composites. Biomaterials. 2008;29:1750–61. https://doi.org/10.1016/j.biomaterials.2007.12.040.
Karpov M, Laczka M, Leboy PS, Osyczka AM. Sol-gel bioactive glasses support both osteoblast and osteoclast formation from human bone marrow cells. J Biomed Mater Res A. 2008;84a:718–26. https://doi.org/10.1002/jbm.a.31386.
Lossdörfer S, Schwartz Z, Lohmann CH, Greenspan DC, Ranly DM, Boyan BD. Osteoblast response to bioactive glasses in vitro correlates with inorganic phosphate content. Biomaterials. 2004;25:2547–55. https://doi.org/10.1016/j.biomaterials.2003.09.094.
Meyer MB, Benkusky NA, Pike JW. The RUNX2 cistrome in osteoblasts: characterization, down-regulation following differentiation, and relationship to gene expression. J Biol Chem. 2014;289:16016–31. https://doi.org/10.1074/jbc.M114.552216.
Li CM, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–24. https://doi.org/10.1016/j.biomaterials.2006.01.022.
Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17:319–36. https://doi.org/10.1007/s00198-005-2035-9.
Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11:1–18. https://doi.org/10.1089/ten.2005.11.1.
Ozeki M, Kuroda S, Kon K, Kasugai S. Differentiation of bone marrow stromal cells into osteoblasts in a self-assembling peptide hydrogel: in vitro and in vivo studies. J Biomater Appl. 2011;25:663–84. https://doi.org/10.1177/0885328209356328.
de Oliveira AAR, de Souza DA, Dias LLS, de Carvalho SM, Mansur HS, Pereira MD. Synthesis, characterization and cytocompatibility of spherical bioactive glass nanoparticles for potential hard tissue engineering applications. Biomed Mater. 2013;8:025011. https://doi.org/10.1088/1748-6041/8/2/025011.
Lei B, Chen XF, Wang YJ, Zhao NR, Du C, Fang LM. Surface nanoscale patterning of bioactive glass to support cellular growth and differentiation. J Biomed Mater Res A. 2010;94a:1091–9. https://doi.org/10.1002/jbm.a.32776.
Baier RE, Dutton RC. Initial events in interactions of blood with a foreign surface. J Biomed Mater Res. 1969;3:191–206. https://doi.org/10.1002/jbm.820030115.
Acknowledgements
This study was funded by grants from the National Key Research and Development Program of China (2016YFA0201704/2016YFA0201700), the Postdoctoral Science Foundation of Jiangsu Province (1701163B), the National Natural Science Foundation of China (81701025), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (2014-37). All authors thank Xiaofeng Chen project group of the South China University of Technology for kindly providing bioactive glass.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Yan, J., Hu, X. et al. Effect of nanoscale bioactive glass with radial spherical particles on osteogenic differentiation of rat bone marrow mesenchymal stem cells. J Mater Sci: Mater Med 31, 29 (2020). https://doi.org/10.1007/s10856-020-06368-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-020-06368-8