Abstract
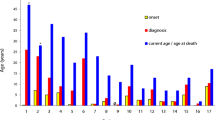
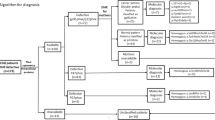
Chronic granulomatous disease (CGD) is a group of inherited disorder of phagocytes, resulting from mutations in the components of the NADPH oxidase complex. Reduced or absent oxygen radical synthesis seen in these patients leads to impaired killing of intracellular bacteria and fungi. CGD clinically presents with recurrent and life-threatening infections as well as granulomatous inflammatory responses. p47phox encoded by the NCF1 gene is the most common autosomal recessive form of CGD which is often clinically milder. Here, we are presenting the data on clinical and immunological findings in 21 Indian patients with Del GT mutation in the NCF1 gene. Diagnosis of these patients was based on detailed clinical evaluation, measurement of respiratory burst activity by nitro blue tetrazolium and dihydrorhodamine-1,2,3 assay, expression of p47phox by flow cytometry, and molecular confirmation by GeneScan method. Seventeen male and four female patients with median age of onset of 1 year ranging from 1.5 months to 6 years were included in the study. Sixty-two percent (13 out of 21) of patients belonged to a consanguineous marriage with only one family having a history of a previous sibling death. Significant variability in clinical presentation was observed in spite of identical genetic defect ranging from asymptomatic to very severe presentation leading to early death or requiring transplantation. However, none of these patients showed difference in immunological parameters to account for this variability. Thus, this study highlights the phenotypic heterogeneity seen in these patients with Del GT mutation in the NCF1 gene and its implication in management of these patients.



Similar content being viewed by others
References
Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol. 2001;2(3):211–5.
Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254(5037):1512–5.
Francke U, Hsieh CL, Foellmer BE, Lomax KJ, Malech HL, Leto TL. Genes for two autosomal recessive forms of chronic granulomatous disease assigned to 1q25 (NCF2) and 7q11.23 (NCF1). Am J Hum Genet. 1990;47(3):483–92. PubMed PMID: 2393022, PubMed Central PMCID: PMC1683885.
Winkelstein JA, Marino MC, Johnston Jr RB, Boyle J, Curnutte J, Gallin JI, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore). 2000;79(3):155–69.
Rawat A, Singh S, Suri D, Gupta A, Saikia B, Minz RW, et al. Chronic granulomatous disease: two decades of experience from a tertiary care centre in North West India. J ClinImmunol. 2014;34(1):58–67.
Jones LB, Mcgrogan P, Flood TJ, Gennery AR, Morton L, Thrasher A, et al. Special article: chronic granulomatous disease in the United Kingdom and Ireland: a comprehensive national patient-based registry. Clin ExpImmunol. 2008;152(2):211–8.
Nunoi H, Ishibashi F. Statistical evaluation of chronic granulomatous disease in Japan and basic studies for gene therapy for CGD patients. Rinsho Byori. 1999;47(7):658–64.
Wolach B, Gavrieli R, de Boer M, Gottesman G, Ben-Ari J, Rottem M, et al. Chronic granulomatous disease in Israel: clinical, functional and molecular studies of 38 patients. Clin Immunol. 2008;129(1):103–14.
El Kares R, Barbouche MR, Elloumi-Zghal H, Bejaoui M, Chemli J, Mellouli F, et al. Genetic and mutational heterogeneity of autosomal recessive chronic granulomatous disease in Tunisia. J Hum Genet. 2006;51(10):887–95.
Agudelo-Flórez P, Prando-Andrade CC, López JA, Costa-Carvalho BT, Quezada A, Espinosa FJ, et al. Chronic granulomatous disease in Latin American patients: clinical spectrum and molecular genetics. Pediatr Blood Cancer. 2006;46(2):243–52.
Al-Zadjali S, Al-Tamemi S, Elnour I, Alkindi S, Lapoumeroulie C, Al-Maamari S, et al. Clinical and molecular findings of chronic granulomatous disease in Oman: family studies. Clin Genet. 2015;87(2):185–9.
Matute JD, Arias AA, Wright NA, Wrobel I, Waterhouse CC, Li XJ, et al. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood. 2009;114(15):3309–15.
Köker MY, Camcıoğlu Y, van Leeuwen K, Kılıç SŞ, Barlan I, Yılmaz M, et al. Clinical, functional, and genetic characterization of chronic granulomatous disease in 89 Turkish patients. J Allergy Clin Immunol. 2013;132(5):1156–63.
Roesler J, Curnutte JT, Rae J, Barrett D, Patino P, Chanock SJ, et al. Recombination events between the p47-phox gene and its highly homologous pseudogenes are the main cause of autosomal recessive chronic granulomatous disease. Blood. 2000;95(6):2150–6.
Noack D, Rae J, Cross AR, Ellis BA, Newburger PE, Curnutte JT, et al. Autosomal recessive chronic granulomatous disease caused by defects in NCF-1, the gene encoding the phagocyte p47-phox: mutations not arising in the NCF-1 pseudogenes. Blood. 2001;97(1):305–11.
Dekker J, de Boer M, Roos D. Gene-scan method for the recognition of carriers and patients with p47(phox)-deficient autosomal recessive chronic granulomatous disease. Exp Hematol. 2001;29(11):1319–25.
Roos D, Kuhns DB, Maddalena A, Bustamante J, Kannengiesser C, de Boer M, et al. Hematologically important mutations: the autosomal recessive forms of chronic granulomatous disease (second update). Blood Cells Mol Dis. 2010;44(4):291–9. doi:10.1016/j.bcmd.2010.01.009. PubMed PMID: 20167518, PubMed Central PMCID: PMC4568122, Epub 2010 Feb 18. Review.
Hayrapetyan A, Dencher PC, van Leeuwen K, de Boer M, Roos D. Different unequal cross-over events between NCF1 and its pseudogenes in autosomal p47(phox)-deficient chronic granulomatous disease. BiochimBiophysActa. 2013 Oct;1832(10):1662–72.
Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363(27):2600–10.
Wada T, Muraoka M, Toma T, Imai T, Shigemura T, Agematsu K, et al. Rapid detection of intracellular p47phox and p67phox by flow cytometry; useful screening tests for chronic granulomatous disease. J Clin Immunol. 2013;33(4):857–64.
Roos D, de Boer M, Köker MY, Dekker J, Singh-Gupta V, Ahlin A, et al. Chronic granulomatous disease caused by mutations other than the common GT deletion in NCF1, the gene encoding the p47phox component of the phagocyte NADPH oxidase. Hum Mutat. 2006;27(12):1218–29.
Gathmann B, Grimbacher B, Beauté J, et al. ESID Registry Working Party. The European Internet-based patient and research database for primary immunodeficiencies: results 2006–2008. Clin Exp Immunol. 2009;157 suppl 1:3–11.
Joshi AY, Iyer VN, Hagan JB, St Sauver JL, Boyce TG. Incidence and temporal trends of primary immunodeficiency: a population-based cohort study. Mayo Clin Proc. 2009;84(1):16–22.
Carnide EG, Jacob CA, Castro AM, Pastorino AC. Clinical and laboratory aspects of chronic granulomatous disease in description of eighteen patients. Pediatr Allergy Immunol. 2005;16(1):5–9.
Madkaikar M, Mishra A, Desai M, Gupta M, Mhatre S, Ghosh K. Comprehensive report of primary immunodeficiency disorders from a tertiary care center in India. J Clin Immunol. 2013;33(3):507–12.
Verma S, Sharma PK, Sivanandan S, Rana N, Saini S, Lodha R, et al. Spectrum of primary immune deficiency at a tertiary care hospital. Indian J Pediatr. 2008;75(2):143–8.
Vowells SJ, Fleisher TA, Sekhsaria S, Alling DW, Maguire TE, Malech HL. Genotype-dependent variability in flow cytometric evaluation of reduced nicotinamide adenine dinucleotide phosphate oxidase function in patients with chronic granulomatous disease. J Pediatr. 1996;128:104–7.
Casimir CM, Bu-Ghanim HA, Rodaway ARF, Bentley DL, Rowe P, Segal AW. Autosomal recessive chronic granulomatous disease caused by deletion at a dinucleotide repeat. Proc Natl Acad Sci USA. 1991;88:2753–7.
Heyworth PG, Noack D, Cross AR. Identification of a novel NCF-1 (p47-phox) pseudogene not containing the signature GT deletion: significance for A47 degrees chronic granulomatous disease carrier detection. Blood. 2002;100(5):1845–51.
Marciano BE, Spalding C, Fitzgerald A, Mann D, Brown T, Osgood S, et al. Common severe infections in chronic granulomatous disease. Clin Infect Dis. 2015;60(8):1176–83. doi:10.1093/cid/ciu1154. Epub 2014 Dec 23. PubMed PMID: 25537876; PubMed Central PMCID: PMC4400412.
Meshaal S, El Hawary R, Abdelaziz D, Alkady R, Galal N, Boutros J, et al. Chronic granulomatous disease: review of a cohort of Egyptian patients. Allergol Immunopathol (Madr). 2015;43(3):279–85.
Marciano BE, Rosenzweig SD, Kleiner DE, Anderson VL, Darnell DN, Anaya-O’Brien S, et al. Gastrointestinal involvement in chronic granulomatous disease. Pediatrics. 2004;114:462–8. PubMed: 15286231.
Freudenberg F, Wintergerst U, Roesen-Wolff A, Albert MH, Prell C, Strahm B, et al. Therapeutic strategy in p47-phox deficient chronic granulomatous disease presenting as inflammatory bowel disease. J Allergy Clin Immunol. 2010;125(4):943–6. doi:10.1016/j.jaci.2010.01.035.
Roos D, de Boer M. Molecular diagnosis of chronic granulomatous disease. Clin Exp Immunol. 2014;175(2):139–49. doi:10.1111/cei.12202. PubMed PMID: 24016250, PubMed Central PMCID: PMC3892405, Review.
Goldblatt D. Recent advances in chronic granulomatous disease. J Infect. 2014;69 Suppl 1:S32–5. doi:10.1016/j.jinf.2014.07.013. Epub 2014 Sep 26. Review.
Kang EM, Marciano BE, Deravin S, Zarember KA, Holland SM, Malech HL. Chronic granulomatous disease: overview and hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2011;127(6):1319–26. doi:10.1016/j.jaci.2011.03.028. PubMed PMID: 21497887, PubMed Central PMCID: PMC3133927, quiz 1327-8. Epub 2011 Apr 17. Review.
Seger RA. Hematopoietic stem cell transplantation for chronic granulomatous disease. Immunol Allergy Clin North Am. 2010;30(2):195–208. doi:10.1016/j.iac.2010.01.003. Review.
Acknowledgment
We would like to thank the Foundation of Primary Immunodeficiency (FPID) for providing antibodies for NADPH oxidase components. We also thank Prof. Martin de Boer, Sanquin Blood Supply Foundation, Amsterdam, The Netherlands, for his valuable suggestions to study the molecular mutations in this cohort.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Kulkarni, M., Desai, M., Gupta, M. et al. Clinical, Immunological, and Molecular Findings of Patients with p47phox Defect Chronic Granulomatous Disease (CGD) in Indian Families. J Clin Immunol 36, 774–784 (2016). https://doi.org/10.1007/s10875-016-0333-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-016-0333-y