Abstract
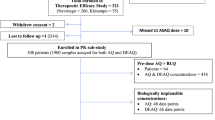
The study aimed to characterize the population pharmacokinetics of amodiaquine (AQ) and its major metabolite N-desethylamodiaquine (N-DEAQ), and to assess the correlation between exposure to N-DEAQ and treatment outcome. Blood samples from children in two studies in Zanzibar and one in Papua New Guinea were included in the pharmacokinetic analysis (n = 86). The children had been treated with AQ in combination with artesunate or sulphadoxine-pyrimethamine. The population pharmacokinetics of AQ and N-DEAQ were modeled using the non-linear mixed effects approach as implemented in NONMEM. Bayesian post-hoc estimates of individual pharmacokinetic parameters were used to generate individual profiles of N-DEAQ exposure. The correlation between N-DEAQ exposure and effect was studied in 212 patients and modeled with logistic regression in NONMEM. The pharmacokinetics of AQ and N-DEAQ were best described by two parallel two-compartment models with a central and a peripheral compartment for each compound. The systemic exposure to AQ was low in comparison to N-DEAQ. The t 1/2λ of N-DEAQ ranged from 3 days to 12 days. There was a statistically significant, yet weak, association between N-DEAQ concentration on day 7 and treatment outcome. The age-based dosing schedule currently recommended in Zanzibar appeared to result in inadequate exposure to N-DEAQ in many patients.
Similar content being viewed by others
References
Murphy SC and Breman JG (2001). Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia and complications of pregnancy. Am J Trop Med Hyg 64(1–2 Suppl): 57–67
Li XQ, Bjorkman A, Andersson TB, Ridderstrom M and Masimirembwa CM (2002). Amodiaquine clearance and its metabolism to N-desethylamodiaquine is mediated by CYP2C8: a new high affinity and turnover enzyme-specific probe substrate. J Pharmacol Exp Ther 300(2): 399–407
Pussard E, Verdier F, Faurisson F, Scherrmann JM, Le Bras J and Blayo MC (1987). Disposition of monodesethylamodiaquine after a single oral dose of amodiaquine and three regimens for prophylaxis against Plasmodium falciparum malaria. Eur J Clin Pharmacol 33(4): 409–414
Winstanley P, Edwards G, Orme M and Breckenridge A (1987). The disposition of amodiaquine in man after oral administration. Br J Clin Pharmacol 23(1): 1–7
Winstanley PA, Simooya O, Kofi-Ekue JM, Walker O, Salako LA, Edwards G, Orme ML and Breckenridge AM (1990). The disposition of amodiaquine in Zambians and Nigerians with malaria. Br J Clin Pharmacol 29(6): 695–701
White NJ, Looareesuwan S, Edwards G, Phillips RE, Karbwang J, Nicholl DD, Bunch C and Warrell DA (1987). Pharmacokinetics of intravenous amodiaquine. Br J Clin Pharmacol 23(2): 127–135
Laurent F, Saivin S, Chretien P, Magnaval JF, Peyron F, Sqalli A, Tufenkji AE, Coulais Y, Baba H and Campistron G (1993). Pharmacokinetic and pharmacodynamic study of amodiaquine and its two metabolites after a single oral dose in human volunteers. Arzneimittelforschung 43(5): 612–616
Gerstner U, Prajakwong S, Wiedermann G, Sirichaisinthop J, Wernsdorfer G and Wernsdorfer WH (2003). Comparison of the in-vitro activity of amodiaquine and its main metabolite, monodesethyl-amodiaquine, in Plasmodium falciparum. Wien Klin Wochenschr 115(Suppl 3): 33–38
Childs GE, Boudreau EF, Milhous WK, Wimonwattratee T, Pooyindee N, Pang L and Davidson DE (1989). A comparison of the in vitro activities of amodiaquine and desethylamodiaquine against isolates of Plasmodium falciparum. Am J Trop Med Hyg 40(1): 7–11
Mariga ST, Gil JP, Sisowath C, Wernsdorfer WH and Bjorkman A (2004). Synergism between amodiaquine and its major metabolite, desethylamodiaquine, against Plasmodium falciparum in vitro. Antimicrob Agents Chemother 48(11): 4089–4096
Aubouy A, Bakary M, Keundjian A, Mbomat B, Makita JR, Migot-Nabias F, Cot M, Le Bras J and Deloron P (2003). Combination of drug level measurement and parasite genotyping data for improved assessment of amodiaquine and sulfadoxine-pyrimethamine efficacies in treating Plasmodium falciparum malaria in Gabonese children. Antimicrob Agents Chemother 47(1): 231–237
Hombhanje FW, Hwaihwanje I, Tsukahara T, Saruwatari J, Nakagawa M, Osawa H, Paniu MM, Takahashi N, Lum JK, Aumora B, Masta A, Sapuri M, Kobayakawa T, Kaneko A and Ishizaki T (2005). The disposition of oral amodiaquine in Papua New Guinean children with falciparum malaria. Br J Clin Pharmacol 59(3): 298–301
Lindegårdh N, Forslund M, Green MD, Kaneko A and Bergqvist Y (2002). Automated solid-phase extraction for determination of amodiaquine, chloroquine and metabolites in Capillary blood on sampling paper by liquid chromatography. Chromatographia 55: 5–12
Jonsson EN and Karlsson MO (1999). Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58(1): 51–64
Beal SL and Sheiner LB (1982). Estimating population kinetics. Crit Rev Biomed Eng 8(3): 195–222
Wahlby U, Jonsson EN and Karlsson MO (2001). Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn 28(3): 231–252
Gobburu JV and Lawrence J (2002). Application of resampling techniques to estimate exact significance levels for covariate selection during nonlinear mixed effects model building: some inferences. Pharm Res 19(1): 92–98
Holford N (2005) A degenerative predictive check. In: 14th P.A.G.E. Meeting. Pamplona
Montgomery DC, Peck EA and Vining GG (2001). Introduction to linear regression analysis. Wiley, Chichester, New York
Holford N (2007) Wings for NONMEM Version 600. Accessed: April 2007
Mihaly GW, Nicholl DD, Edwards G, Ward SA, Orme ML, Warrell DA and Breckenridge AM (1985). High-performance liquid chromatographic analysis of amodiaquine in human plasma. J Chromatogr 337(1): 166–171
Wennerholm A, Nordmark A, Pihlsgard M, Mahindi M, Bertilsson L and Gustafsson LL (2006). Amodiaquine, its desethylated metabolite, or both, inhibit the metabolism of debrisoquine (CYP2D6) and losartan (CYP2C9) in vivo. Eur J Clin Pharmacol 62(7): 539–546
Rowland M and Tozer TN (1995). Clinical pharmacokinetics: concepts and applications. Williams and Wilkins, USA
Guidelines for the treatment of malaria/ World Health Organization 2006
Taylor WR, Terlouw DJ, Olliaro PL, White NJ, Brasseur P and ter Kuile FO (2006). Use of weight-for-age-data to optimize tablet strength and dosing regimens for a new fixed-dose artesunate-amodiaquine combination for treating falciparum malaria. Bull World Health Organ 84(12): 956–964
Martensson A, Stromberg J, Sisowath C, Msellem MI, Gil JP, Montgomery SM, Olliaro P, Ali AS and Bjorkman A (2005). Efficacy of artesunate plus amodiaquine versus that of artemether-lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, Tanzania. Clin Infect Dis 41(8): 1079–1086
Bukirwa H, Yeka A, Kamya MR, Talisuna A, Banek K, Bakyaita N, Rwakimari JB, Rosenthal PJ, Wabwire-Mangen F, Dorsey G and Staedke SG (2006). Artemisinin combination therapies for treatment of uncomplicated malaria in Uganda. PLoS Clin Trials 1(1): e7
Eggelte TA, Agtmael MA and Boxtel CJ (1999). Artemisinin drugs in the treatment of malaria: from medicinal herb to registered medication. Trends Pharmacol Sci 20(5): 199–205
Taylor WR, Rigal J and Olliaro PL (2003). Drug resistant falciparum malaria and the use of artesunate-based combinations: focus on clinical trials sponsored by TDR. J Vector Borne Dis 40(3–4): 65–72
Borrmann S, Adegnika AA, Missinou MA, Binder RK, Issifou S, Schindler A, Matsiegui PB, Kun JF, Krishna S, Lell B and Kremsner PG (2003). Short-course artesunate treatment of uncomplicated Plasmodium falciparum malaria in Gabon. Antimicrob Agents Chemother 47(3): 901–904
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hietala, S.F., Bhattarai, A., Msellem, M. et al. Population pharmacokinetics of amodiaquine and desethylamodiaquine in pediatric patients with uncomplicated falciparum malaria. J Pharmacokinet Pharmacodyn 34, 669–686 (2007). https://doi.org/10.1007/s10928-007-9064-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-007-9064-2