Abstract
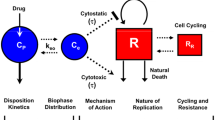
Combination therapies are widely used in the treatment of patients with cancer. Selecting synergistic combination strategies is a great challenge during early drug development. Here, we present a pharmacokinetic/pharmacodynamic (PK/PD) model with a smooth nonlinear growth function to characterize and quantify anticancer effect of combination therapies using time-dependent data. To describe the pharmacological effect of combination therapy, an interaction term was introduced into a semi-mechanistic anticancer PK/PD model. This approach enables testing of a pharmacological hypothesis with respect to an anticipated pharmacological synergy of drug combinations, such as an assumed pharmacological synergy of complementary inhibition of a particular signaling pathway. The model was applied to three real data sets derived from preclinical screening experiments using xenograft mice. The suggested model fitted well the observed data from mono- to combination-therapy and allowed physiologically meaningful interpretation of the experiments. The tested drug combinations were assessed for their ability to act as synergistic modulators of tumor growth inhibition by the interaction parameter ψ. The presented approach has practical implications because it can be applied to standard xenograft experiments and may assist in the selection of both optimal drug combinations and administration schedules. The unique feature of the presented approach is the ability to characterize the nature of combined drug interaction as well as to quantify the intensity of such interactions by assessing the time course of combined drug effect.





Similar content being viewed by others
References
Sun YN, Jusko WJ (1998) Transit compartments versus gamma distribution function to model signal transduction processes in pharmacodynamics. J Pharm Sci 87:732–737
Mager DE, Wyska E, Jusko WJ (2003) Diversity of mechanism-based pharmacodynamic models. Drug Metab Dispos 31:510–518
Lobo ED, Balthasar JP (2002) Pharmacodynamic modeling of chemotherapeutic effects: application of a transit compartment model to characterize methotrexate effects in vitro. AAPS Pharm Sci 4:E42
Simeoni M, Magni P, Cammia C, De Nicolao G, Croci V, Pesenti E, Germani M, Poggesi I, Rocchetti M (2004) Predictive pharmacokinetic–pharmacodynamic modeling of tumor growth kinetics in xenograft models after administration of anticancer agents. Cancer Res 64:1094–1101
Rocchetti M, Poggesi I, Germani M, Fiorentini F, Pellizzoni C, Zugnoni P, Pesenti E, Simeoni M, De Nicolao G (2005) A pharmacokinetic–pharmacodynamic model for predicting tumour growth inhibition in mice: a useful tool in oncology drug development. Basic Clin Pharmacol Toxicol 96:265–268
Magni P, Simeoni M, Poggesi I, Rocchetti M, De Nicolao G (2006) A mathematical model to study the effects of drugs administration on tumor growth dynamics. Math Biosci 200:127–151
Rocchetti M, Simeoni M, Pesenti E, De Nicolao G, Poggesi I (2007) Predicting the active doses in humans from animal studies: a novel approach in oncology. Eur J Cancer 43:1862–1868
Berenbaum MC (1989) What is synergy? Pharmacol Rev 41:93–141
Greco WR, Bravo G, Parsons JC (1995) The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 47:331–385
Jusko WJ (1971) Pharmacodynamics of chemotherapeutic effects: dose-time-response relationships for phase-nonspecific agents. J Pharm Sci 60:892–895
Magni P, Germani M, De Nicolao G, Bianchini G, Simeoni M, Poggesi I, Rochetti M (2008) A Minimal Model of Tumor Growth Inhibition. IEEE Biomedical Eng 55(12):2683–2690
Chakraborty A, Jusko WJ (2002) Pharmacodynamic interaction of recombinant human interleukin-10 and prednisolone using in vitro whole blood lymphocyte proliferation. J Pharm Sci 91:1334–1342
D’Argenio DZ, Schumitzky A (1997) ADAPT II user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedica simulation resuource, Los Angeles
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Drewinko B, Loo TL, Brown B, Gottlieb JA, Freireich EJ (1976) Combination chemotherapy in vitro with adriamycin. Observations of additive, antagonistic, and synergistic effects when used in two-drug combinations on cultured human lymphoma cells. Cancer Biochem Biophys 1:187–195
Greco WR, Park HS, Rustum YM (1990) Application of a new approach for the quantitation of drug synergism to the combination of cis-diamminedichloroplatinum and 1-beta-D-arabinofuranosylcytosine. Cancer Res 50:5318–5327
Berenbaum MC (1990) Re: W. R. Greco et al., application of a new approach for the quantitation of drug synergism to the combination of cis-diamminedichloroplatinum and 1-beta-d-arabinofuranosylcytosine. Cancer Res 50:5318–5327. Cancer Res 52:4558–4560 (1992)
Loewe S, Muischnek H (1926) Effect of combinations: mathematical basis of problem. Arch Exp Pathol Pharmakol 114:313–326
Hairer E, Nørsett SP, Wanner G (1993) Solving ordinary differential equations I, Chapter I. vol 14, 2nd edn. Springer, USA
Acknowledgments
The authors are grateful to WJ Jusko for motivating and valuable discussion on this modeling approach and to Karl Zech who continuously supported the project. The authors thank the scientists Petra Gimmnich, Astrid Zimmermann, Ragna Hussong, and Rolf Herzog for their dedicated involvement during the generation of pharmacological and pharmacokinetic data, and Kathy B. Thomas for helpful suggestions during the preparation of the manuscript. This study was sponsored by Nycomed GmbH, Konstanz, Germany.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Appendix 1: The unperturbed growth function
In the Simeoni model, the unperturbed tumor growth function is modeled by a linear and a constant part
which are simply glued together. Here \( \lambda_{0} > 0 \) and \( \lambda_{1} > 0 \) describe the rate of exponential and linear growth of tumor weight w. Moreover the growth function g suffers at a lack of differentiability for \( w = w_{th} . \)
Alternatively, we model the unperturbed tumor growth by the term
To achieve physiological meaningful parameters with the same meaning as in Simeoni’s model we adjust actual values of a and b according to
These two conditions ensure that the maximal growth rate and the half maximum value of the Simeoni growth function g and f coincide.
We obtain \( a = \lambda_{1} ,b = \frac{{\lambda_{1} }}{{2\lambda_{0} }} \) and end up with the expression
Remark: The growth function g from Simeoni et al. (Eq. 7) is not differentiable at the switch w th therefore they suggested an approximation
with ψ = 20 fixed. A comparison of the growth functions is shown in Fig. 6.
Appendix 2: The threshold concentration
In this section we give a proof of (Eq. 4) and calculate a similar result for combination therapy. Let the drug flow be constant for all t ≥ 0. The stationary equations of the presented model (Eq. 3) and (Eq. 6) read:
with
(Eq. 8b) and (Eq. 8c) directly imply
Inserting the relation (Eq. 9) into (Eq. 8a) leads to the quadratic equation
for γ. We set x 1 = 0 in (Eq. 10) due to the eradication of the tumor and find the unique positive solution
of (Eq. 10). Thus, with \( \tau = \frac{N - 1}{{k_{1} }} \) we obtain for the monotherapy model
and for the combination therapy system
Moreover, it is confirmed in both cases by numerical simulation that the domain of attraction of the steady state (0,…,0) covers \( {\mathbb{R}}_{ + }^{N} . \)
Appendix 3: Derivatives with respect to parameters
In this section we present the concept of variations, that is, an alternative approach to compute partial derivatives of solutions of ordinary differential equations with respect to parameters. With this approach the user can balance accuracy of the computations versus computational effort. Please keep in mind that the accurate computation of these partial derivatives affects PK/PD modeling for two reasons. First it opens the route to use more powerful numerical algorithms to solve nonlinear least squares problems and, secondly, it can be useful to reliably compute the covariance matrix, which is the basis for every a posteriori stochastic analysis.
The model equations of tumor development in PK/PD analysis have the general form
with \( \theta = \left( {\theta_{1} ,\theta_{2} ,\theta_{3} ,\theta_{4} } \right) \) and with the differentiable solution \( \overline{x} \left( {t,x_{0} ,\theta } \right). \) To calculate the derivatives \( \frac{{\partial \overline{x} }}{{\partial \theta_{k} }}\left( {t,x_{0} ,\theta } \right),k = 1, \ldots ,4 \) due to Hairer [19] one has to solve the matrix differential equation
and \( \frac{{\partial \overline{x} }}{{\partial w_{0} }}\left( {t,x_{0} ,\theta } \right) \) is the solution of
In (Eq. 12), \( {\mathbb{R}}^{N,4} \) stands for the space of matrices with N rows and 4 columns. To actually compute the derivatives one has to solve (Eq. 11), (Eq. 12) and (Eq. 13) simultaneously with an appropriate numerical scheme. Note that (Eq. 12) has to be solved column by column.
Rights and permissions
About this article
Cite this article
Koch, G., Walz, A., Lahu, G. et al. Modeling of tumor growth and anticancer effects of combination therapy. J Pharmacokinet Pharmacodyn 36, 179–197 (2009). https://doi.org/10.1007/s10928-009-9117-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-009-9117-9