Abstract
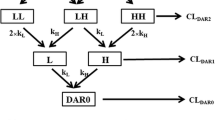
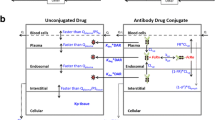
Antibody–drug conjugate (ADC) is a complex structure composed of an antibody linked to several molecules of a biologically active cytotoxic drug. The number of ADC compounds in clinical development now exceeds 30, with two of them already on the market. However, there is no rigorous mechanistic model that describes pharmacokinetic (PK) properties of these compounds. PK modeling of ADCs is even more complicated than that of other biologics as the model should describe distribution, binding, and elimination of antibodies with different toxin load, and also the deconjugation process and PK of the released toxin. This work extends the target-mediated drug disposition (TMDD) model to describe ADCs, derives the rapid binding (quasi-equilibrium), quasi-steady-state, and Michaelis–Menten approximations of the TMDD model as applied to ADCs, derives the TMDD model and its approximations for ADCs with load-independent properties, and discusses further simplifications of the system under various assumptions. The developed models are shown to describe data simulated from the available clinical population PK models of trastuzumab emtansine (T-DM1), one of the two currently approved ADCs. Identifiability of model parameters is also discussed and illustrated on the simulated T-DM1 examples.





Similar content being viewed by others
References
Mullard Asher (2013) Maturing antibody–drug conjugate pipeline hits 30. Nat Rev Drug Discovery 12:329–332
Lin Tibbitts (2012) Pharmacokinetic considerations for antibody drug conjugates. Pharm Res 29:2353–2366
Leipold D, Bender B, Xu K, Theil F-P, and Tibbitts J (2009) Understanding the de-conjugation of Trastuzumab-MCC-DM1 through application of a multi-compartmental model of individual drug: antibody species in cynomolgus monkey. Presented at the 2009 American Association for Cancer Research (AACR) Meeting, Denver, Colorado
Bender B, Leipold D, Liu L, Xu K, Shen B-Q, Friberg LE, Tibbitts J. A multicompartmental population PK model elucidating the complex disposition of trastuzumab emtansine (T-DM1): an antibody-drug conjugate for the treatment of HER2-positive cancer, Page 21 (2012) Abstr 2607 [www.page-meeting.org/?abstract=2607]
Lu D, Joshi A, Wang B, Olsen S, Yi JH, Krop IE, Burris HA, Sandhya Girish S, An integrated multiple-analyte pharmacokinetic model to characterize trastuzumab emtansine (T-DM1) clearance pathways and to evaluate reduced pharmacokinetic sampling in patients with HER2-positive metastatic breast cancer. Clin Pharmacokinet 52(8):657-672
Chudasama VL, Schaedeli Stark F, Harrold JM, Tibbitts L, Girish SR, Gupta M, Frey N, Mager DE (2012) Semi-mechanistic population pharmacokinetic model of multivalent trastuzumab emtansine in patients with metastatic breast cancer. Clin Pharmacol Ther 92(4):520–527. doi:10.1038/clpt.2012.153
Mager DE, Jusko WJ (2001) General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 28:507–532
Mager DE, Krzyzanski W (2005) Quasi-equilibrium pharmacokinetic model for drugs exhibiting target-mediated drug disposition. Pharm Res 22(10):1589–1596
Gibiansky L, Gibiansky E, Kakkar T, Ma P (2008) Approximations of the target-mediated drug disposition model and identifiability of model parameters. J Pharmacokinet Pharmacodyn 35(5):573–591
Gibiansky L, Gibiansky E (2009) Target-mediated drug disposition model: approximations, identifiability of model parameters, and applications to the population pharmacokinetic–pharmacodynamic modeling of biologics. Expert Opin Drug Metabol Toxicol 5(7):803–812
Yan X, Mager DE, Krzyzanski W (2010) Selection between Michaelis-Menten and target-mediated drug disposition pharmacokinetic models. J Pharmacokinet Pharmacodyn 37(1):25–47
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gibiansky, L., Gibiansky, E. Target-mediated drug disposition model and its approximations for antibody–drug conjugates. J Pharmacokinet Pharmacodyn 41, 35–47 (2014). https://doi.org/10.1007/s10928-013-9344-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-013-9344-y